Dermal Services
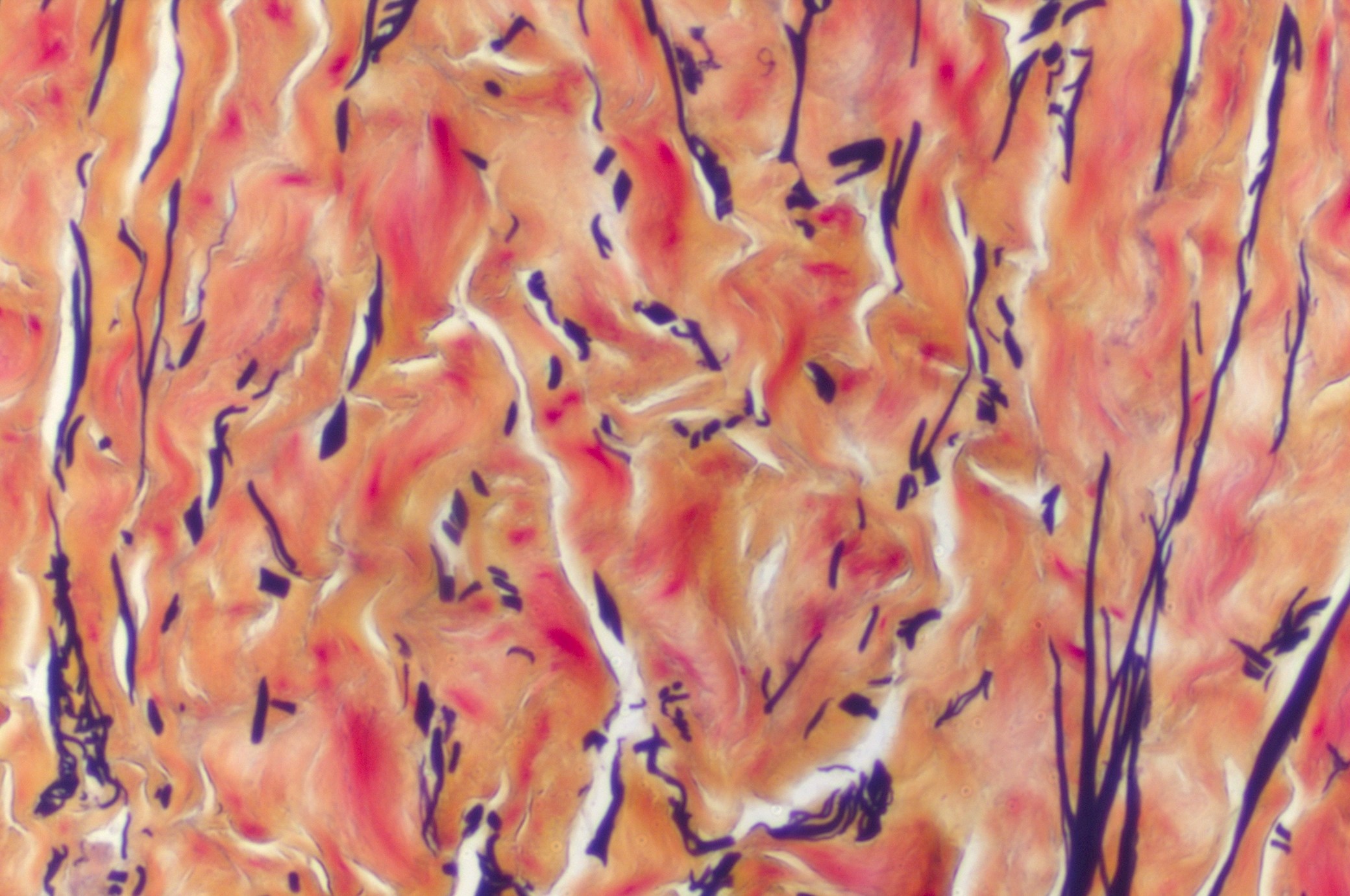
LifeNet Health provides an array of in vitro services designed to assess the potential for chemical, corrosion, irritation and sensitization when applied to the skin. In addition to global harmonization standards (GHS) hazard evaluation, which follow OECD test guidelines, we also provide potency assessments that allow you to evaluate the degree to which a test material may cause damage to the skin. For more mechanistic assessments, cytokine release, (e.g. IL-1α), can be performed to determine potential inflammatory responses. We also offer a histopathology option to provide a visual depiction of the degree and depth of injury to the epidermis. Custom services for quantitative assessment of dermal sensitization are also available.
LifeNet Health offers a dermal absorption assay, that is performed according to test guidelines, OECD 428 or the EUs scientific committee on consumer safety (SCCS) guidance documentation. Options for customized detection methodologies for analytes not compatible with LC/MS/MS, and customized skin parameters (e.g. age, phototype, race, sex) are also available.
We are privileged to provide this service with skin donated through our anatomical gifting program.
Connect with an expert
Tests We Offer
- Dermal Corrosion – OECD 431 or OECD 435 Corrositex®
- Dermal Irritation – Hazard Assessment (OECD 439)
- Dermal Irritation – Potency Assessment (ET50 method)
- Skin Sensitization – DPRA/OECD 442C, KeratinoSens(OECD442D)
- Skin Sensitization Testing (USENS) Assay
- Dermal Absorption – OECD 428 or SCCS/1358/10
Expert Regulatory Guidance
- FDA
- EPA
- Health Canada
- European Food Safety Association
- European Medicines Agency
Our Process Sets Us Apart From Other Laboratories
Consultation – We partner with you to determine the best approach, offering customized study plans that draw from our 60-plus years of combined in vitro testing experience. We may recommend utilizing more than one assay when performing a thorough cytotoxicity screening.
Assay Development – We develop and validate your assay using many different cell models, with an emphasis on all-human primary cells and biospecimens to simulate in vitro conditions, which provides more relevant, reliable results than traditional testing methods. This is part of our commitment to advancing alternative methods to support organizations in moving away from traditional animal-based models.
Data Analysis – We provide expert data analysis, including offering adaptable options when needed based on early results.
Results Review – Our clear, concise reports bring simplicity to your biggest data challenges, putting your organization in the best position for regulatory reviews. We may suggest follow-up studies to further your organization’s research goals.
Our facilities are compliant with Good Laboratory Practices and Good In Vitro Method Practices (OECD 286).
