TruVivo® 2D+ Hepatic System
TruVivo is the first and only 2D+ hepatic system to provide human-relevant, reliable results in an easy-to-use format. The pioneering 2D+ system combines the best features of 2D and 3D models. With TruVivo, users can expect the simplicity and flexibility of a 2D model, along with the relevance, longevity, architectural integrity, and robustness of a 3D model.
This combination of attributes makes TruVivo a valuable tool in ADME/DMPK, toxicology, and disease modeling applications for the pharmaceutical and agriscience industries. TruVivo kits include prequalified primary human hepatocytes and feeder cells combined at an optimized ratio to mimic the microarchitecture and functionality of the human liver, along with optimized media.
For clients who want to leave the work to us, TruVivo is also available as a service — an option that offers true simplicity and ease. Our team of expert scientists can use TruVivo in cytotoxicity studies, ADME/DMPK assays, or to represent the liver in our multi-organ platform screening service.
- Combines the simplicity and flexibility of a 2D model, with the relevance, longevity, architectural integrity, and robustness of a 3D model
- Self-assembled hepatocytes maintain physiologic hepatocellular morphology and architecture, mimicking native hepatocytes
- Viability and physiologically-relevant hepatic protein expression is maintained
- Native hepatocyte functionality, metabolic capacity, and integrity maintained long-term
- Enables prolonged chemical exposures, or re-exposures, as necessary, through extended culture
- Stable morphology and basic functionality for at least 2 weeks
- Hepatocyte colony formation
- Hepatocyte confluency ≥50% on day 14
- Albumin secretion 26-105 µg/day/106 hepatocytes on days 7-14
- Urea secretion 39-159 µg/day/106 hepatocytes on days 7-14
TruVivo's unique combination of relevance, reliability, and simplicity make it ideal for the widest range of potential applications, including
- Metabolism of low clearance compounds
- Drug-induced liver injury (DILI)
- Disease modeling
- Toxicity testing
- High content imaging
A Pioneering 2D+ Hepatic System
Predicting therapeutic safety and efficacy is an important part of preclinical drug development, but many of the current preclinical models either lack phenotypic stability and longevity, fail to mimic human hepatic functionality, or are too complex and time-consuming to use. LifeNet Health LifeSciences has developed TruVivo, a new, all-human 2D+ hepatic system for 96- and 24-well plates to help address these challenges .
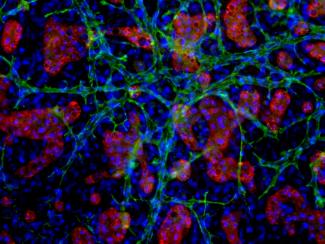
TruVivo mimics the microarchitecture and basic functionality of the human liver, allowing for greater relevance and reliability. This system is comprised entirely of primary human cells, including endothelial and stromal feeder cells (FCs) with primary human hepatocytes (PHHs). The primary hepatocytes self-assemble into multicellular colonies along with a mixed feeder cell population (Figure 1) - offering extended viability and architectural integrity, physiological relevant albumin and urea production, and stable transporter and metabolic capacity for at least two weeks. This longevity and human relevance in culture, as well as high-throughput capability, makes TruVivo an ideal platform for drug discovery and development applications that require long-term and repeated chemical exposures.
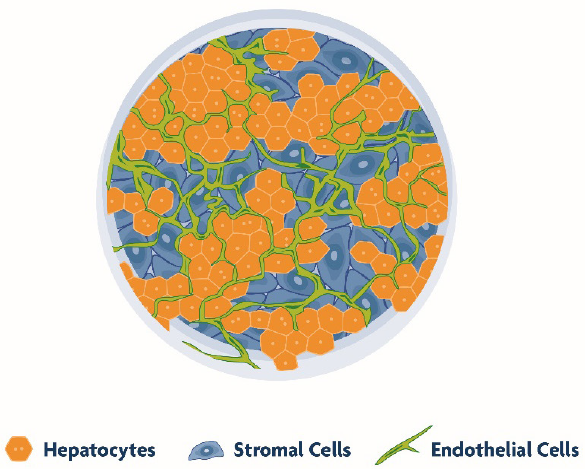
TruVivo Mimics Key Features of Human Liver
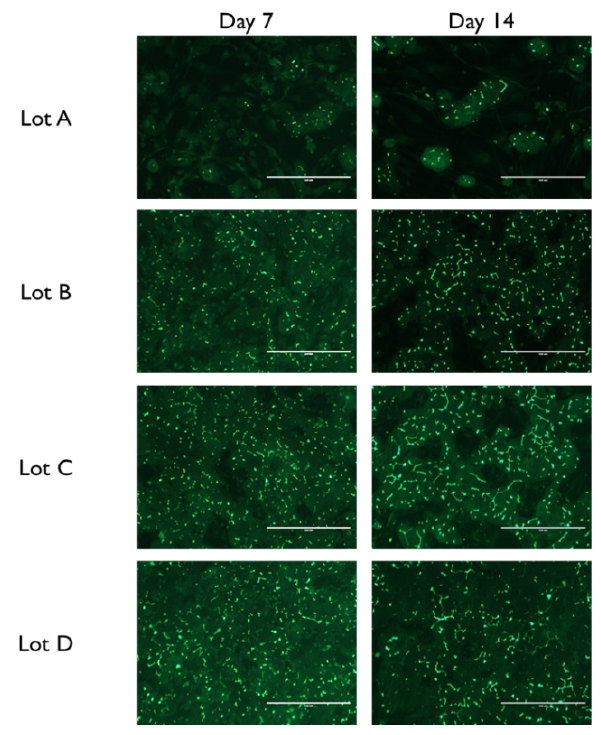
Prequalified primary human hepatocytes cultured together with human endothelial and stromal cells in the TruVivo system retain their native cuboidal morphology and self-assemble into distinct multicellular colonies with well-defined borders through two weeks (Figure 2).
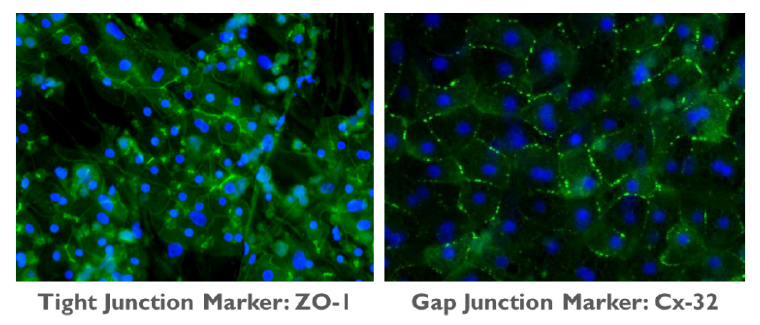
Within these hepatocyte colonies, the PHHs form cell-cell interactions and junctional complexes, as indicated by positive staining for CX-32 and ZO-1, which are markers for gap junctions and tight junctions, respectively (Figure 3). Extensive functional bile canalicular networks form between the PHHs, the extent of which increases over time through 14 days in culture (Figure 4). Collectively, these results demonstrate that PHHs in TruVivo self-assemble and mimic native human hepatocyte properties.
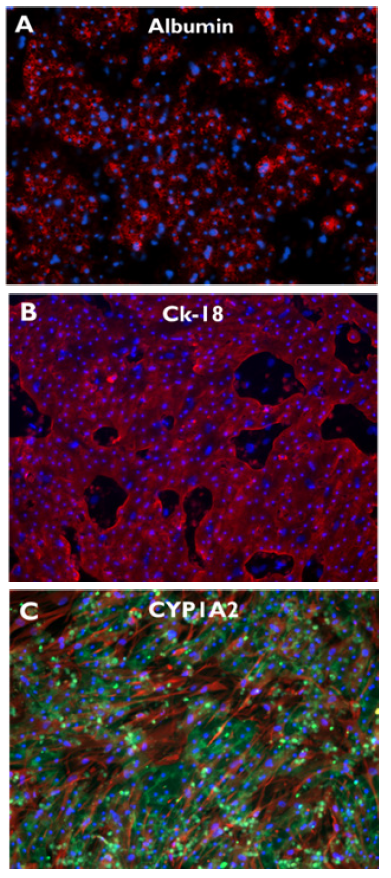
Further, robust expression of key liver-specific proteins was detected in PHHs cultured in TruVivo through at least 14 days, including albumin and CYP1A2 (Figure 5). Additionally, CK-18, an epithelial cytoskeletal marker protein, was likewise detected through 14 days, and highlights the clearly defined borders of the hepatocyte colonies. Collectively, these results demonstrate the retention of native hepatic protein expression in PHHs through 14 days of culture in the system.
TruVivo Maintains Human Hepatic Functionality and Culture Longevity
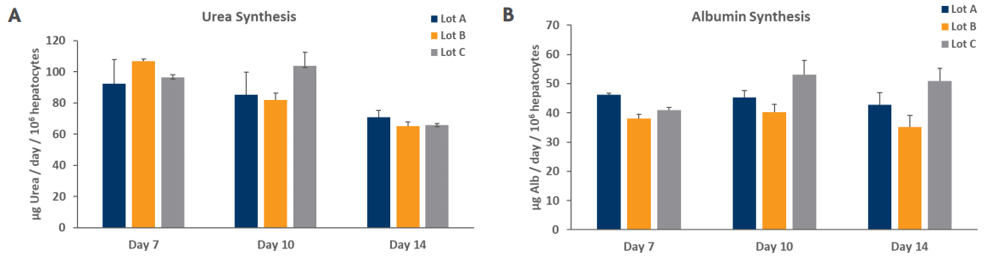
As a further measure of hepatocyte phenotype, native hepatocyte function of PHHs in the TruVivo system was evaluated by measuring urea and albumin secretion levels, which were shown to be within physiologic levels of 56-159 and 37-105 µg/day/106 hepatocytes, respectively, as reported previously1 (Figure 6). Notably, these physiologically relevant levels were stable and consistent through a minimum of 14 days in culture across multiple lots.
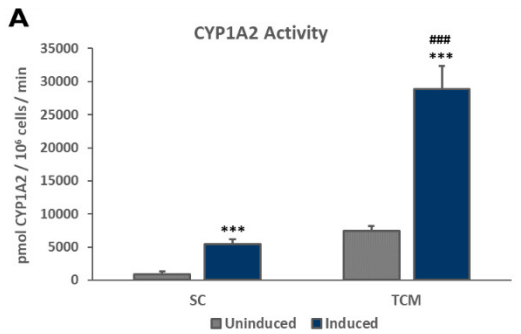
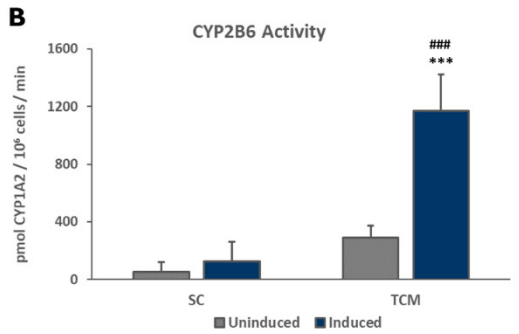
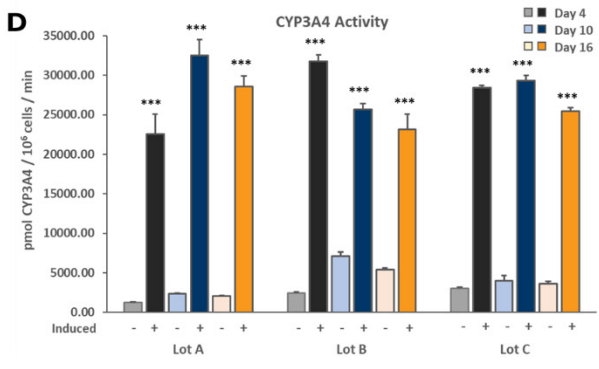
Members of the Cytochrome P450 (CYP450) enzyme family are responsible for the liver’s metabolism of the majority of known therapeutic drugs.2 Drug-drug interactions due to receptor-mediated increases in CYP expression can be an important consequence of exposure to receptor agonists. Therefore, the stability of the induction capacity of PHHs cultured in the TruVivo system versus traditional sandwich cultures was determined by evaluating the induced activity of key CYP450 enzymes. For PHHs cultured in the TruVivo system, there was a significant increase in induced levels of CYP1A2, CYP2B6, and CYP3A4 compared to uninduced levels, with no detectable background signal from feeder cells, and in induced CYP1A2 and CYP3A4 levels in PHHs in sandwich cultures (Figure 7). Notably, the induced levels of CYP3A4 were similar in both culture models, whereas the induced levels of CYP1A2 and CYP2B6 were significantly higher in TruVivo compared to those in the sandwich culture. The induced CYP3A4 activity in PHHs in TruVivo was very stable and reproducible across multiple lots through 16 days in culture. Therefore, PHHs cultured in TruVivo maintain their metabolic capacity and response to inducers that is higher and more stable over time compared traditional sandwich cultures.
Summary
LifeNet Health’s all-human TruVivo system mimics the microarchitecture and functionality of the human liver, offering a more physiologically relevant in vitro liver model versus alternative in vitro and in vivo models. By combining primary human hepatocytes with human endothelial and stromal cells, TruVivo effectively maintains viability and hepatic functionality over long-term culture. LifeNet Health’s all-human TruVivo system represents a promising new alternative method for chemical screening under more physiologic conditions.
References
- Baudy, A.R., et al. Liver microphysiological systems development guidelines for safety risk assessment in the pharmaceutical industry. Lab on a Chip, 2020. 20(2): p. 215-225.
- Wienkers, L.C. and T.G. Heath. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov, 2005. 4(10): p. 825-33.
