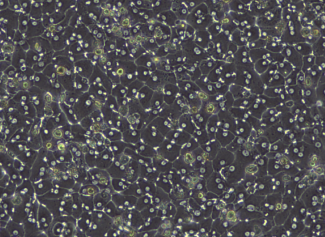
Primary Human Hepatocytes
LifeNet Health LifeSciences offers a large inventory of primary human hepatocytes in plateable and suspension formats. Inventory includes induction qualified and 3D spheroid-qualified lots.
Hepatocytes make up approximately 60% of all cells within the liver and perform multiple functions including protein synthesis and storage, carbohydrate metabolism, lipid metabolism, and detoxification. Primary human hepatocytes are the gold standard for metabolism, drug-drug interaction, and toxicity studies.
LifeNet Health is a supplier of MPS-validated hepatocyte and Kupffer cells for use in CN Bio’s PhysioMimix® organ-on-a-chip range of MPS. Reach out to us to learn more.
- Comprehensive donor medical and social history provided
- Donor-matched cells and tissue samples
- Large inventory with average lot size >450 vials
- Histopathology report includes Non-Alcoholic Fatty Liver Disease (NAFLD) activity score (NAS) along with H&E and Trichrome stained images
- All lots genotyped for pharmacologically relevant drug metabolizing enzymes and liver disease single nucleotide polymorphisms
- Select lots tested for 3D spheroid formation
- Access to technical expertise and guidance from LifeSciences’ team of scientists
- Optimized Hepatocyte Thawing, Plating, and Culture Media
- Viability ≥70%
- Yield ≥5.0 X 10⁶ cells/vial
- Phase I and II Enzyme Activity
- Histopathology assessment of tissue of origin by board-certified pathologist; NAS* and fibrosis stage** provided
- Induction of CYP3A4, CYP2B6, and CYP1A2 mRNA and specific activity for adult mid- and long-term plateable categories
- For spheroid-qualified batches: 3D spheroids formation that remains viable for at least 21 days with ATP measurements on days 7, 14, and 21
- Vials of cryopreserved cells shipped and stored at ≤-135°C
*NAFLD (Non-Alcoholic Fatty Liver Disease) score (NAS) was assigned according to the standards of the NASH CRN Scoring System (Hepatology 41: 1313-1321, 2005).
**Inflammation and fibrosis were assessed using standard Batts-Ludwig scoring methodology (Scale, 0-4, American Journal of Surgical Pathology 19: 1409-1417, 1995).
Human Hepatocytes are suitable for a range of applications, including
- Metabolic clearance
- Drug-Drug interaction
- Disease modeling
- Transporter assays
- Pharmacogenomics studies
- 2D and 3D cell culture
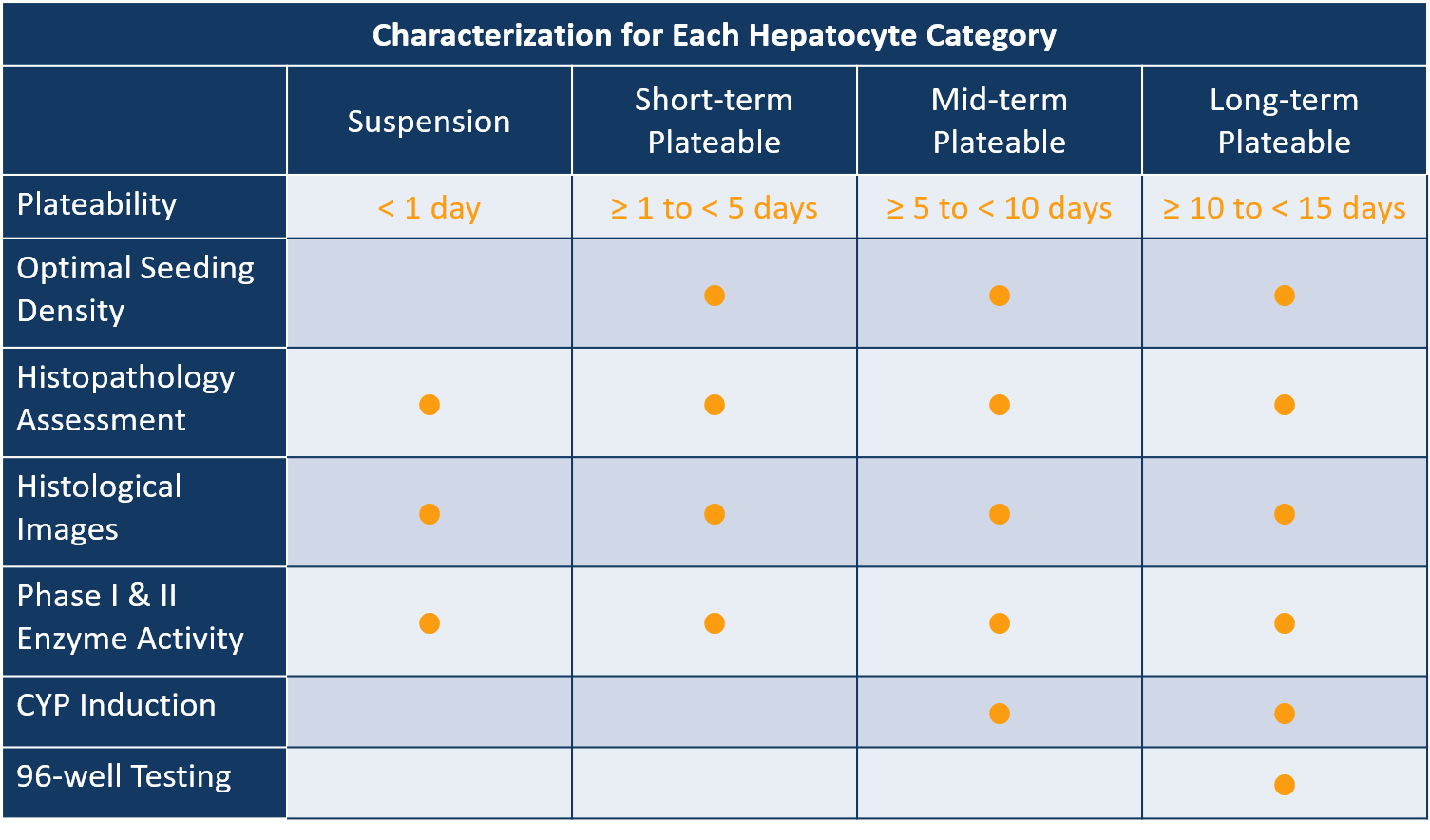
Comprehensive Portfolio of Liver Cells
200+
Lots
0-6
Nas Scores
0-4
Fibrosis Stages
What are Hepatocytes
Hepatocytes are specialized epithelial cells that represent nearly 80% of the total liver mass. These liver cells are polygonal in shape with well-defined borders, granular cytoplasm, and one or more large, centrally located nuclei. They exhibit highly polarized architecture and organization of the cytoplasm and plasma membrane in order to fulfill the multitude of metabolic, endocrine, and secretory tasks they are required to perform.
The Function of Hepatocytes
Hepatocytes perform the majority of the biochemical and physiological functions associated with the liver, including the metabolism of carbohydrates, lipids, and xenobiotics. Hepatocytes are also responsible for the synthesis of key binding and carrier proteins in the blood and the major enzymes and transporter proteins involved in the uptake, metabolism, and efflux of xenobiotics and their metabolites.
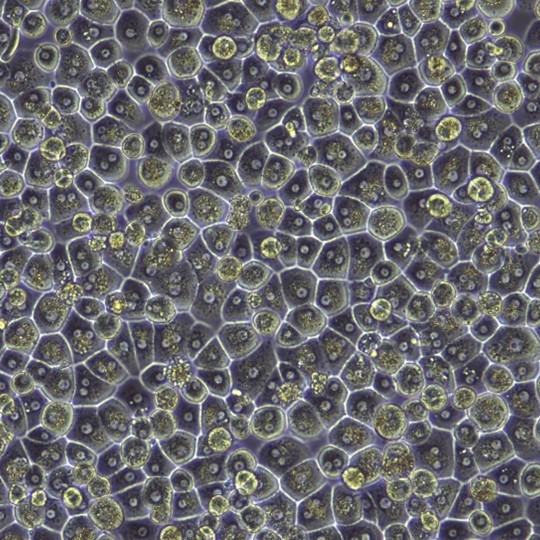
Current Isolation Practices and the Impact of Hepatocyte Profiles
Most protocols utilized today for the isolation of primary human hepatocytes are based on the original two-step methods developed by Berry and Friend (1969) and by Seglen et al. (1976). These protocols rely on the use of crude, ill-defined preparations of collagenase and non-specific proteases. In addition, further enrichment using Percoll density gradient centrifugation is used almost without exception (Kreamer et al., 1986; LeCluyse et al., 1996). These methods when adapted for the isolation of primary human hepatocytes (LeCluyse et al., 2005; LeCluyse and Alexandre, 2010) produce batches of cells that vary in their composition and phenotype, as they represent a mixture of zonal hepatocyte subpopulations with varying amounts of “contaminating” non-parenchymal cells.
Because hepatocytes are a direct reflection of the source tissue and culture environment, LifeNet Health LifeSciences procures these cells using state-of-the-art conditions utilizing enhanced tissue flushing and preservation methods, which minimize warm and cold ischemia times as well as the time from recovery to laboratory. The extraordinary level of care and quality control used in the preparation of our preclinical tissue and cellular products are a result of the processing technologies LifeNet Health has developed and improved throughout its nearly 40-year history in regenerative medicine.
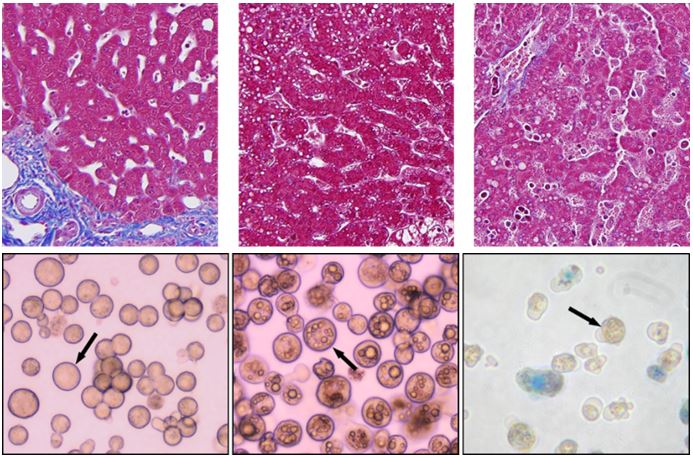
The overall impact of these additional measures minimizes the inherent biological variability and process-related artificial variability that negatively impact hepatocyte quality. The image below reveals variations in source tissue and resulting hepatocytes when biological differences, such as disease status, or damaged tissue caused by poor handling and processing are present.
Applications of Primary Human Hepatocytes
Primary human hepatocytes are widely accepted as the gold standard for in vitro evaluation of metabolism, drug-drug interactions, drug transporter activity, and toxicity of drug candidates.
In the pharmaceutical industry, multiple in vitro hepatocyte culture strategies are utilized in the early phases of drug development. For example, suspension assays can be used to predict the pharmacokinetics and clearance of compounds, while sandwich culture model systems are useful for evaluating drug uptake and efflux transporters or assessing a compound’s potential for adverse interactions through the induction or inhibition of liver enzymes.
More recently, advanced hepatocyte culture systems that are longer lasting and more metabolically stable are being utilized to study drug metabolism and transport as well as drug-induced liver injury in vitro. These long-term primary hepatocyte culture models are particularly useful in addressing long-term clearance of drugs with low turnover rates.
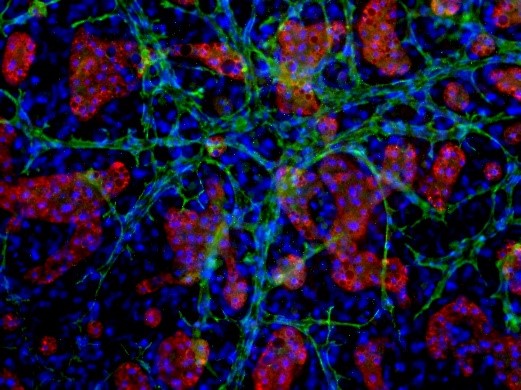
Emerging Hepatocyte Culture Technologies
There is a growing demand for human-based model systems that reflect the complex mechanisms that occur under physiologically relevant exposure conditions. This has prompted an extensive amount of effort on multi-dimensional and multi-cellular in vitro culture systems that retain the innate physiology, metabolic capacity, and adaptive response of native tissues. Current advanced hepatic culture models include co-cultures containing non-parenchymal feeder cells, iPSC-derived cells, spheroid aggregates, 3D bioprinted tissue constructs, microfluidic devices, and bioreactors.
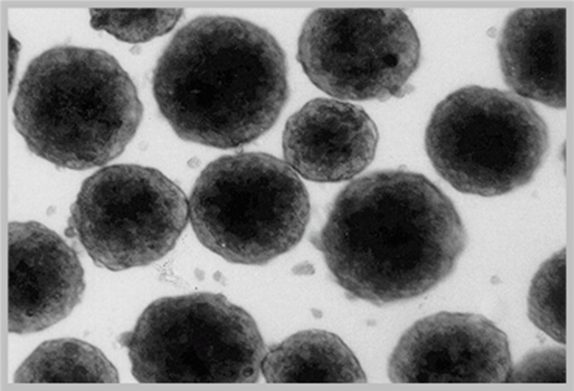
Characterization of LifeNet Health LifeSciences Primary Human Hepatocytes
LifeNet Health LifeSciences provides a comprehensive set of characterization data for each hepatocyte lot so that our customers are equipped with a better understanding about the donor and the tissue from which the cells were derived. This understanding aids in the lot selection process, ultimately reducing time and money spent to determine suitable lots for specific applications.
Donor History: Our comprehensive certificate of analysis (CoA) includes all relevant demographics; blood chemistries; BMI; pertinent drug, alcohol, and tobacco history; and serological test results.
Histopathology: Every tissue is scored by a board-certified pathologist to determine a non-alcoholic fatty liver disease (NAFLD) activity score. Each CoA contains the histopathological scoring as well as H&E and trichrome stained images.
Genotyping: Our hepatocytes are now genotyped for pharmacologically relevant phase I and II enzymes which have a direct impact on the metabolism of drug candidates. Genotyping data includes the actual sequencing call, the reference allele, and the allele frequency of 112 single nucleotide polymorphisms (SNPs) and is customized to the donor’s ethnicity. Based on the genotyping results a classification of poor, intermediate, or extensive (normal) metabolizer can be made. Learn more about genotyping here.
Post-thaw Assessment: Each batch of cryopreserved hepatocytes is carefully characterized to determine the average post-thaw viability and yields per vial, as well as morphological integrity, attachment efficiency, and suitability in cell culture applications. Recommended seeding densities for obtaining optimal confluency is reported for plateable batches. The CoA contains representative images of the cell monolayers in culture.
Metabolic Activity: Each batch is tested for cytochrome P450 (CYP) enzyme activity using prototype selective substrates for CYP1A2, CYP2B6, CYP2C9, CYP2C8, CYP2C19, and CYP3A4, as well as for combined phase I and II metabolism of 7-ethoxycoumarin (7-EC) to 7-hydroxycoumarin (7-HC) and the corresponding sulfated and glucuronidated conjugates.
Induction: Mid- and long-term plateable batches of hepatocytes are tested for response to prototype inducers of CYP1A2, CYP2B6, and CYP3A4 after 48- and 72-hour treatments. Data is reported for mRNA fold change and specific enzyme activity.
3D Spheroid-Qualification: Mid- and long-term plateable batches of hepatocytes are tested for spheroid formation. Spheroids form by day 7 and remain viable for at least 21 days. ATP levels on days 7, 14, and 21 are reported along with spheroid images on days 1, 7, 14, and 21.
Categories of LifeNet Health LifeSciences Primary Human Hepatocytes
Adult High BMI/NAFLD/NASH: Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in Western countries, with a wide disease spectrum. It ranges from the hepatic accumulation of lipids known as steatosis, to non-alcoholic steatohepatitis (NASH, steatosis accompanied by inflammation and fibrosis), or to cirrhosis and hepatocellular carcinoma. Because of our unique network of partner institutions, we have access to a broader range of tissues representing both healthy and diseased status. Accordingly, we are able to provide primary hepatocyte lots from tissue with various stages of NAFLD, including steatosis and NASH.
Neonatal/Pediatric/Juvenile: Developmental changes in the expression of drug metabolism and other clearance mechanisms determine the pharmaco- and toxicokinetics of chemicals at different life stages. These differences are critical in regulating the clearance and accumulation of drugs, and thus influence the pharmaco- and toxico-dynamic responses in newborns and children. Studying these age-related differences is critical for understanding and preventing potential hepatotoxic events.

Adult Suspension and Short-term Plateable: Primary adult human hepatocytes are considered the preferred method for determining the metabolic stability and intrinsic clearance of new compounds in development. Our suspension and short-term plateable hepatocytes are characterized for drug-metabolizing enzyme activity using prototype selective substrates for major phase I and II metabolic pathways. Short-term plateable hepatocytes are guaranteed to maintain stable, confluent monolayers from one to four days.
Adult Mid- and Long-Term Plateable: Use of cultured primary human hepatocytes has become the accepted best practice for conducting in vitro testing of new drugs for their potential to be involved in unwanted drug-drug interactions due to induction of hepatic clearance pathways. Our mid- and long-term plateable hepatocytes are characterized for drug-metabolizing enzyme activity and tested for response to prototype inducers of CYP1A2, CYP2B6, and CYP3A4. Mid-term and long-term plateable hepatocytes are guaranteed to maintain stable, confluent monolayers from five to nine days or ten to fourteen days, respectively.
Frequently Asked Questions:
- Why would you use primary human hepatocytes as opposed to cell lines? Unlike most hepatic cell lines, which by definition are altered or transformed cells, primary hepatocytes retain most, if not all, of their original biochemical and molecular signaling pathways and phenotypic gene expression profiles, including important transcription factors and nuclear receptor responses. These are typically required for more discerning studies to determine human-specific hepatic disposition and hepatic responses to compound exposure, especially species- or population-specific metabolism, uptake and toxicity outcomes.
- Can I repeatedly thaw and refreeze cryopreserved hepatocytes if I only need to remove a small aliquot of cells from a vial? Although theoretically possible, it is not recommended to submit primary hepatocytes to repeated freezing and thawing cycles due to the additional stress and damage imposed on the cells and their subcellular components.
- Is it possible to "plate" cryopreserved human hepatocytes? Yes, but not all batches of cryopreserved hepatocytes will ‘plate’ under standard culture conditions (i.e., collagen-coated 96- or 24-well culture plates). It is important to identify those batches of cells that have been pre-qualified as ‘plateable’ lots based on the data in the individual CoA’s, such as their optimal seeding density, attachment efficiency, formation of confluent monolayers, and viability over time in culture.
- Can I seed hepatocytes in suspension (non-adherent conditions)?Yes, all batches of cryopreserved hepatocytes can be utilized for suspension cultures for short-term, non-adherent experiments, such as drug metabolism or uptake studies. Optimal culture conditions for seeding density, recommended medium and incubation conditions are provided in the Instructions for Use. If experiments require the development of 3D aggregates, such as spheroid cultures, inquire with your technical representative to determine which lots are best suited for these applications.
- What culture vessel do I culture the suspension cells in? If the cells are to be utilized for drug metabolism or uptake experiments, low-binding multi-well plates (i.e., Corning 96-well) are recommended for these applications. In some cases glass vials or culture tubes in a shaking water bath may be used as well.
- How long are these cells viable for? There is significant lot-to-lot variability in how long primary hepatocytes stay viable and retain functionality over time in culture. Longevity and functionality over time in culture is also dependent on the culture conditions and format, such as ECM overlay, medium formulation, co-culture with stromal cells, or 3D aggregate culture. “Standard” monolayer culture conditions, such as ECM overlaid or non-overlaid, support most plateable hepatocyte batches for 1-2 weeks, depending on the lot, ECM composition and the medium formulation. More advanced cell culture systems, including co-cultures with stromal cells or 3D aggregate culture, have been shown to greatly extend the longevity and performance of primary hepatocytes out to several weeks.
- What seeding density should be used for a 96-well or 384-well plate? For plateable hepatocytes, the optimal seeding density for achieving a confluent monolayer is typically between 125,000 and 150,000 cells per cm2. However, each batch of primary human hepatocytes exhibits different attachment efficiencies. Refer to the CoA documents for the optimal seeding density determined for individual plateable lots. The technical bulletins for plateable and suspension lots contain valuable information on the number of cells per incubation using different well formats
- How long would I need to plate the hepatocytes until they are assay ready? For drug metabolism and uptake experiments, the cells are ready for use after attachment (i.e., 2-4 hours). For induction testing, hepatocyte monolayers in standard multi-well plate formats are refractory to drug treatments during the initial 12-18 hours and treatments with drugs are typically started the following day after seeding. For hepatotoxicity and biliary excretion testing, it is recommended that cell-cell adhesions and cellular polarity be restored as much as possible, which can vary somewhat between lots, and experiments should be initiated after several days in culture.
- When I am thawing, re-suspending, and plating primary hepatocytes, can I handle the cells the same way as hepatic cell lines, such as HepG2 and Huh7 cells? Compared to most hepatic cell lines, primary hepatocytes are larger in size, more fragile and generally should be treated more gently while handling. It is recommended that gentle thawing and resuspension methods be adopted when handling cell pellets and during pipetting and plating of hepatocytes. Harsher mechanical resuspension techniques, such as vortexing or rapid mixing with a pipette, should be avoided to preserve the integrity and viability of the cells.
- Do I need to use an ECM overlay, co-culture with other cell types, or other “advanced” culture method for my studies? It is important to understand whether a “simple” culture method, i.e., hepatocyte monolayers on collagen-coated plates, or a more “sophisticated” culture system, i.e., sandwich-culture, co-culture with stromal cells, or 3D spheroid aggregate cultures, is better suited for your particular application(s). For many standard metabolism and uptake experiments, a simple system will often suffice especially if throughput and efficiency is a major consideration. However, the more advanced models usually provide greater longevity, stability and performance over time and biologically relevant outcomes than the simple models. Please consult your technical representative for more information and to discuss your particular application requirements.
- What is the difference between lots that are plateable and suspension? Plateable lots adhere to, and form, confluent monolayers when cultured on collagen-coated plates (24, 48, 96 wells). Plateable lots exhibit ≥ 70% post-thaw viability, ≥ 80% confluency, and ≥ 80% attachment efficiency in adhering to the plate. Suspension lots are not plateable and experiments are typically conducted in a vial, tube, or low binding plate with the cells in suspension culture.
This content was authored, reviewed, and approved by Ph.D. scientists. March 08, 2021.
