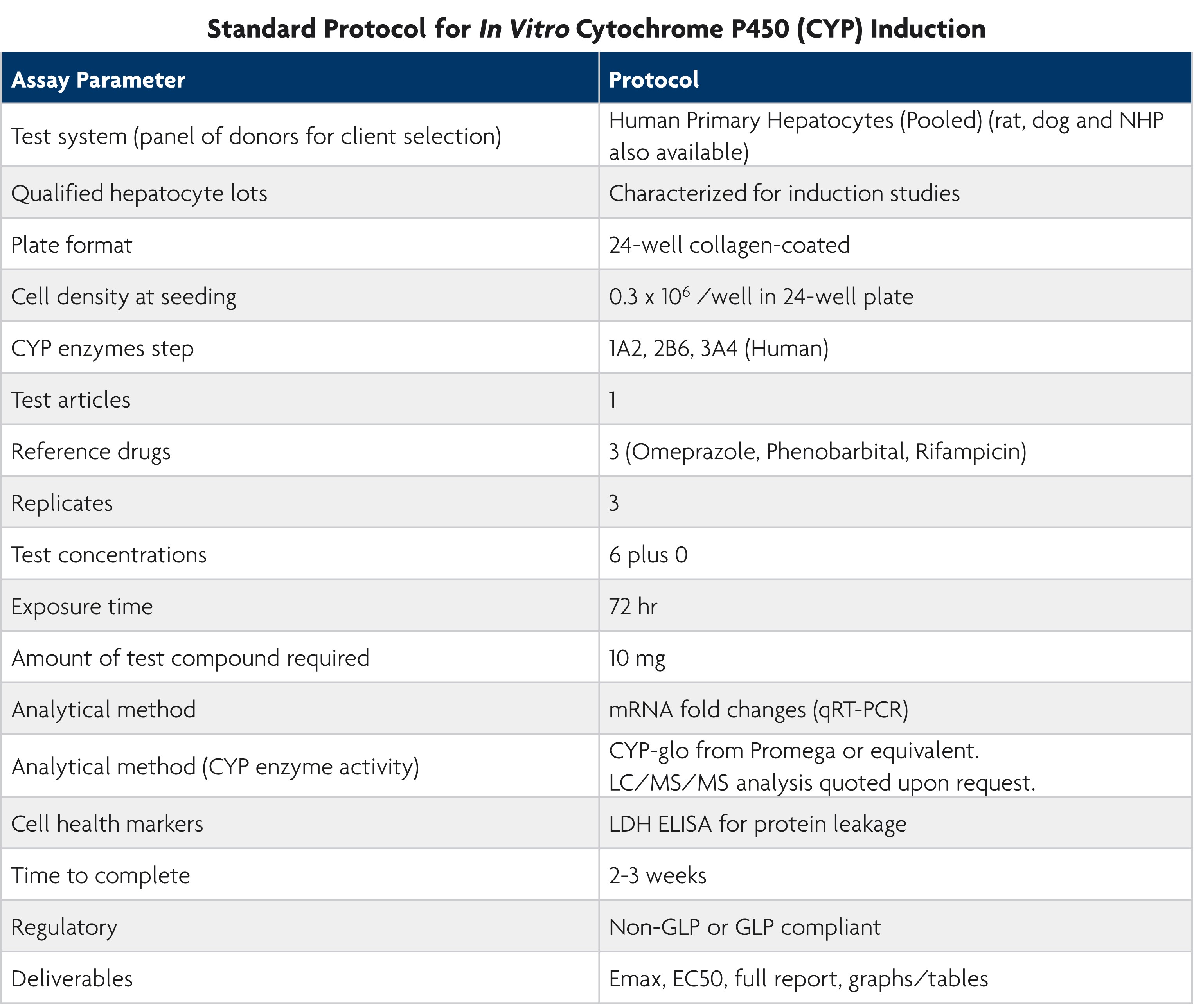
CYP Induction Assay
Evaluating new drug candidates for their potential to induce Cytochrome P450 (CYP) enzymes is an important step in the drug discovery and development process. Our CYP induction assays can help to accurately predict the metabolism of drugs in the body.
Importance of Determining Drug-Mediated Induction of Cytochrome P450 (CYP) Enzymes
- Safety of induced metabolites: CYP induction may lead to changes in the pharmacokinetic profile of a drug, leading to an unexpected and unsafe toxicity level.
- Efficacy of co-medications: The induction of CYP enzymes for one drug may reduce the therapeutic efficacy of other medications in the body.
FDA Guidance Based Methods
LifeNet Health LifeSciences Cell-Based Assays Services offers two CYP inductions assays, both based on the FDA Guidance document entitled, In Vitro Metabolism and Transporter Mediated Drug-Drug Interaction Studies.
The evaluation focuses on the primary CYPs, such as CYP1A2, CYP2B6, and CYP3A4/5, with further assessment of other CPY2C enzymes recommended if significant induction of CYP3A4/5 occurs.
Method 1: Gene Expression Fold-Change
When incubated with the investigational drug, the fold-change in CYP enzyme mRNA levels can be examined by using a cutoff determined from a known positive and negative. Additional parameters, such as EC50 and Maximum induction level (Emax), can also be determined by incorporating detailed concentration response curves.
Method 2: Enzyme Activity
Enzyme activity following induction can be measured by luminescent signals, utilizing the CYP-glo kit. Upon request, the rate of metabolism of a specific substrate can also be determined by the LC/MS/MS method.
Your Partner in CYP Induction Assays
Our services team offers a standardized protocol for assessing both gene expression fold-change and enzyme activity of CYP induction. This ensures high-quality data with the speed and accuracy needed for early drug discovery. We provide study designs suitable for IND submissions and collaborate with you to tailor the study to your specific research needs.
Benefits of Our Services:
- Accurate and reliable data for informed decision-making
- Fast turnaround times to keep your drug development on track
- FDA method-based studies to meet regulatory requirements
- Collaborative approach to ensure the study addresses your research questions
Connect with an expert
