Accurately Predict In Vivo Hepatic Clearance (Clint) with TruVivo®
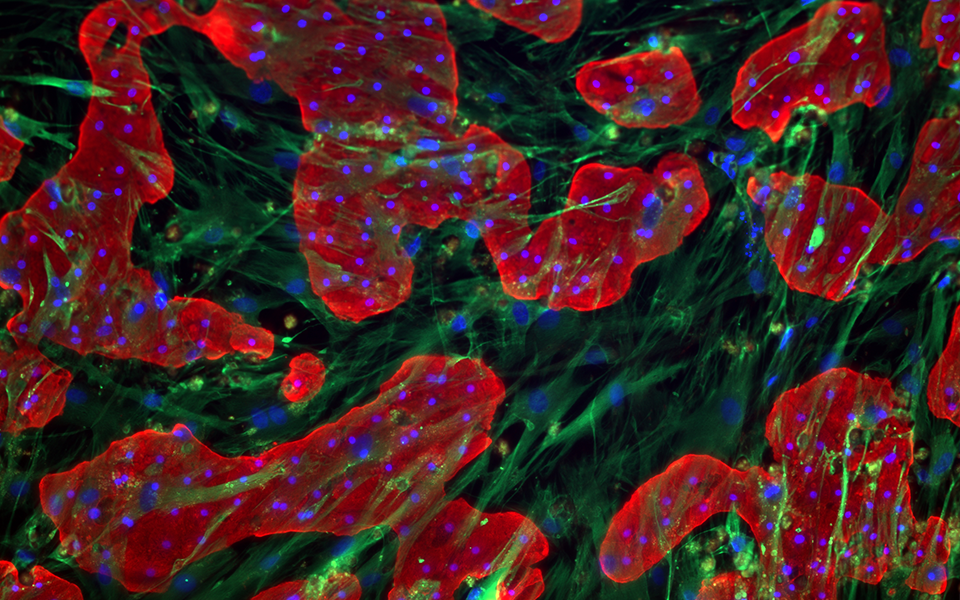
Accurate in vitro prediction of hepatic clearance (Clint) is pivotal for successful drug development. Designing drugs for oral administration with low first-pass metabolism and longer half-lives requires addressing challenges in predicting slow metabolism and low Clint values.
The commonly used substrate depletion method to predict Clint is limited by the absence of phase II metabolism and transporter interactions in current in vitro systems.
While cryopreserved human primary hepatocytes in 2D culture are a gold standard, challenges such as interindividual variability, transporter issues, and prolonged culture times for low Clint molecules persist. Current models are suboptimal for predicting in vivo outcomes, especially for slowly metabolized compounds.
TruVivo
- All-human Cells: TruVivo combines pre-qualified primary human hepatocytes with human feeder cells of stromal and endothelial origin.
- Human Relevance: Hepatocytes in this system maintain their morphology, metabolic health, drug metabolizing capacity (phase I and phase II), and transporter function and orientation for more than 14 days.
- Broad Applicability: TruVivo can provide Cl data for high, intermediate, and low Clint compounds in a single model.
Elevating TruSIMPLICTY to a Service
Our TruVivo service offers true ease and simplicity. Our experts have developed the following standard protocol for assessing Clint in TruVivo, bringing simplicity to your biggest data challenges and allowing you to leave the work to us.
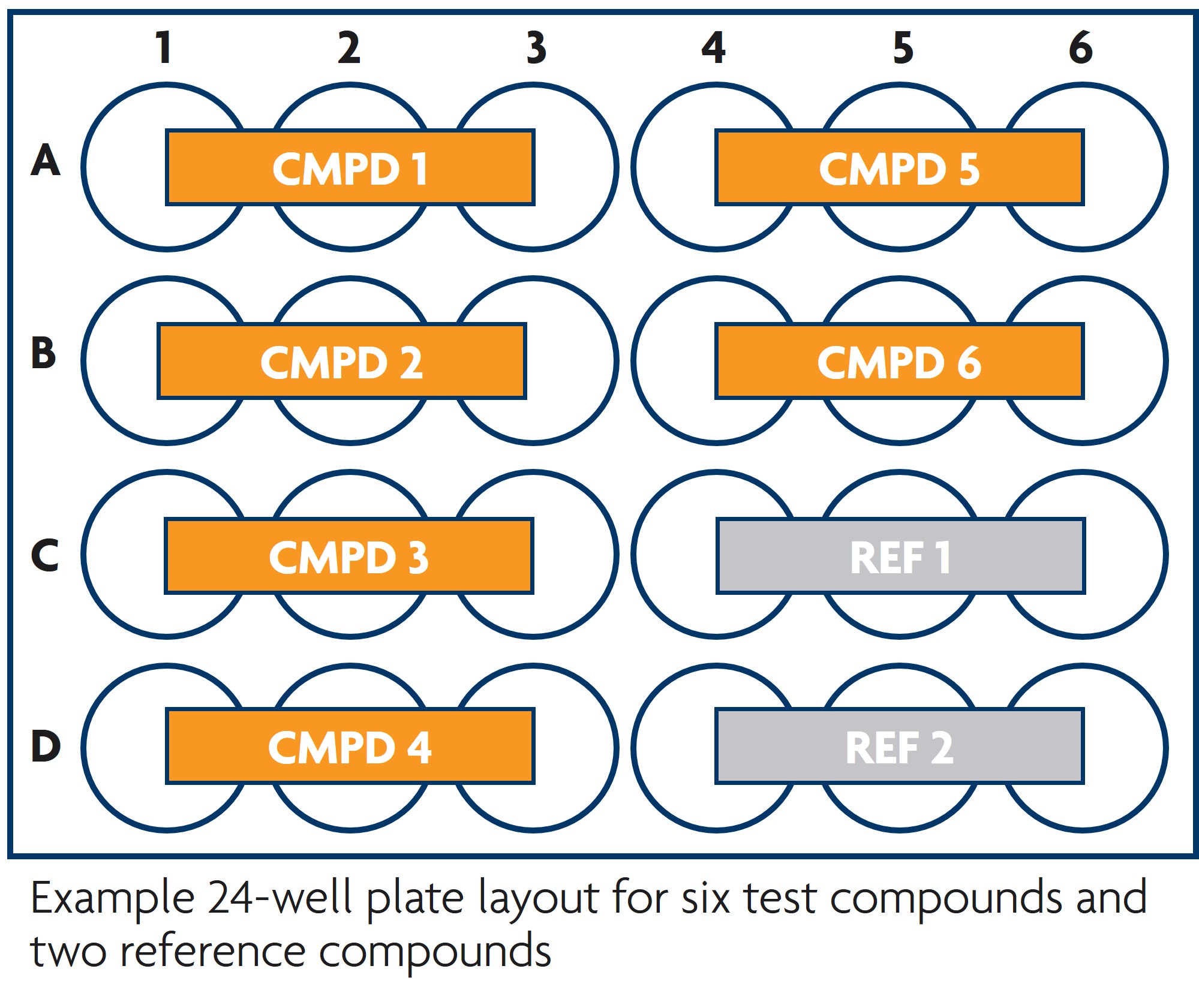
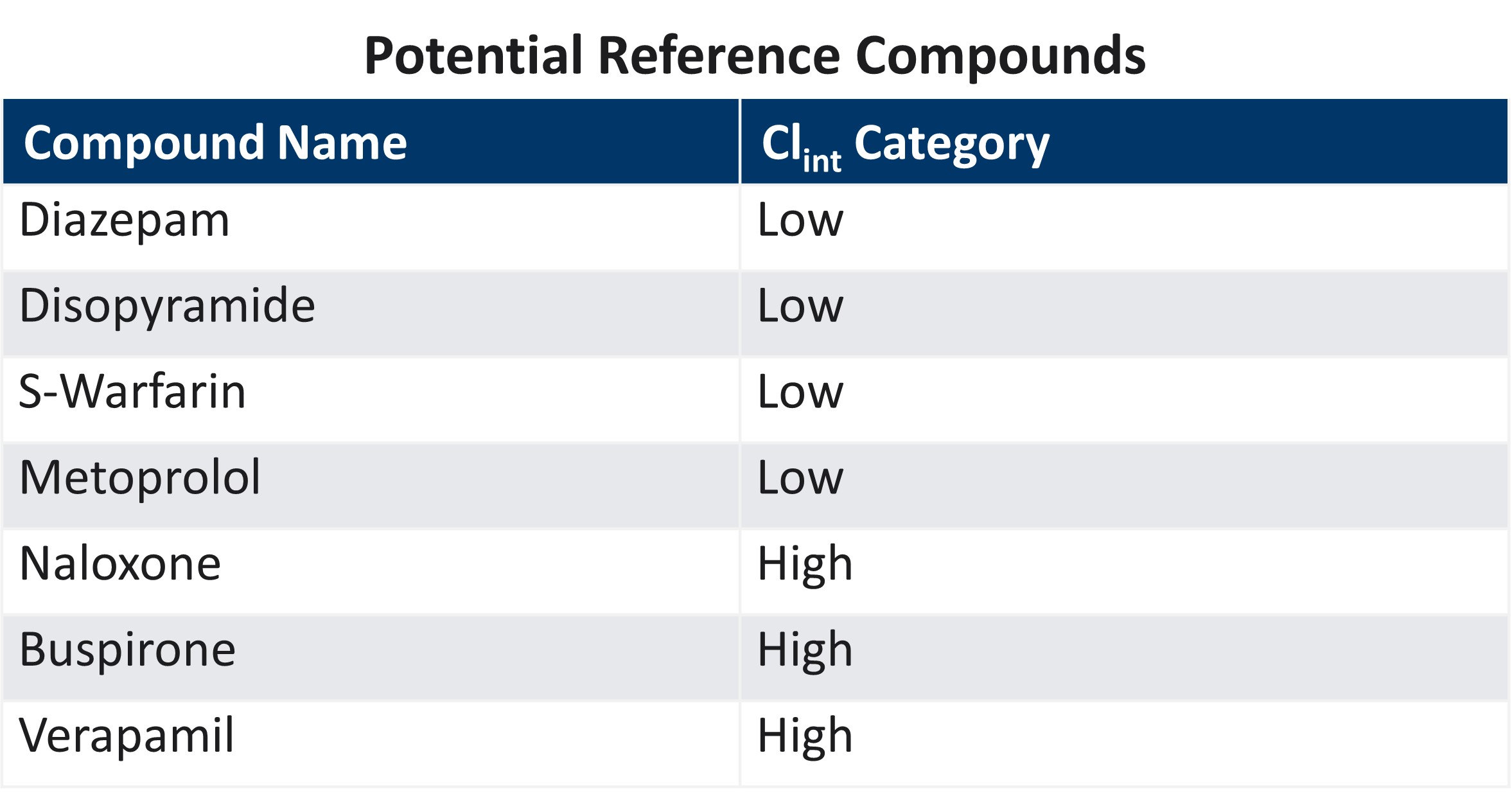
Tests We Offer in TruVivo
- Intrinsic Clearance
- Thyroxine (T4) Metabolism Related to CAR/PXR Activation
- TruVivo + Kupffer Cells
- Hepatoxicity (DILI)
Our Process Sets Us Apart From Other Laboratories
Consultation – We partner with you to determine the best approach, offering study plans that draw from our 60-plus years of combined in vitro testing experience. We may recommend utilizing more than one assay when performing a thorough cytotoxicity screening.
Assay Development – We develop and validate your customized assay using many different cell models, with an emphasis on all-human primary cells and biospecimens to simulate in vitro conditions, which provides more relevant, reliable results than traditional testing methods. This is part of our commitment to advancing alternative methods to support organizations in moving away from traditional animal-based models.
Data Analysis – We provide expert data analysis, including offering adaptable options when needed based on early results.
Results Review – Our clear, concise reports bring simplicity to your biggest data challenges, putting your organization in the best position for regulatory reviews. We may suggest follow-up studies to further your organization’s research goals.
We offer extensive testing capabilities. Our facilities are compliant with Good Laboratory Practices and Good In Vitro Method Practices (OECD 286).
