In Vitro Ocular Irritation Assay
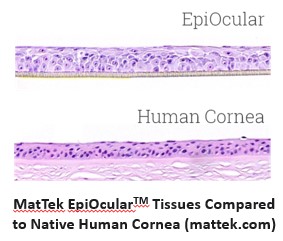
Assessing the risk eye injury is a primary consideration in determining the safety of an ophthalmologic drugs or cosmetics. It is also of concern in the case of accidental exposures to chemicals, formulations, and other products.
The Importance of Early Ocular Testing
- Risk Identification: Early assessment can determine a product's relative risk for causing eye irritation and/or serious eye damage.
- Reduce Further Testing: Initial testing may identify chemicals or mixtures with a low potential for eye injury and obliviate the need to further in vitro or in vivo testing.
Your Partner in Ocular Irritation Assays
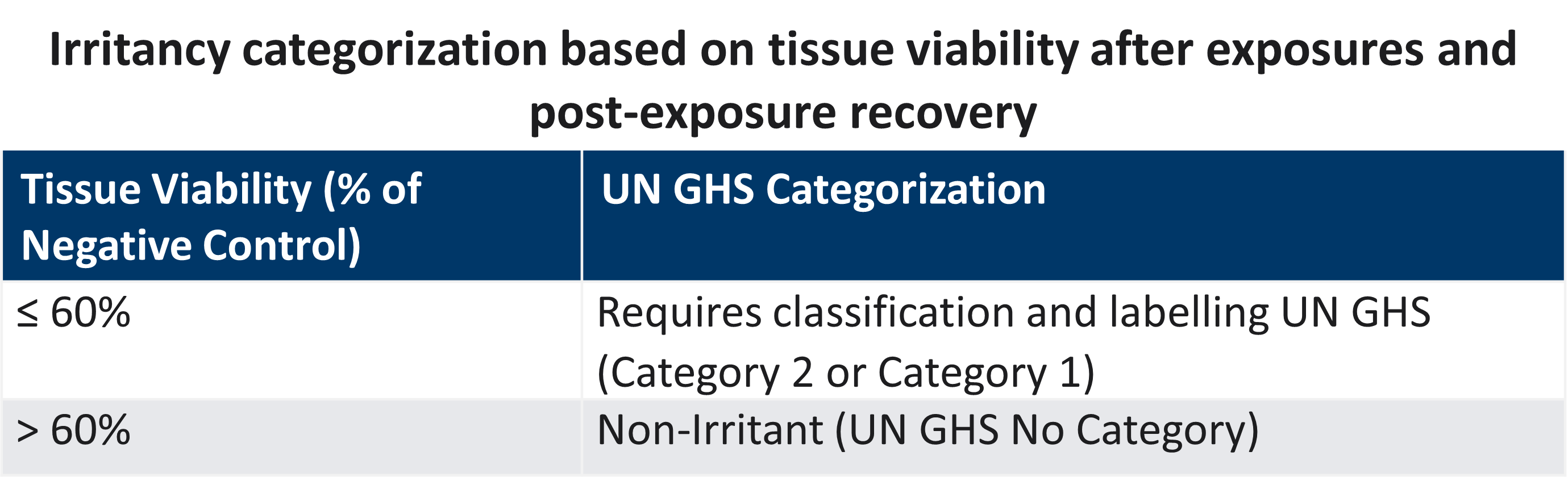
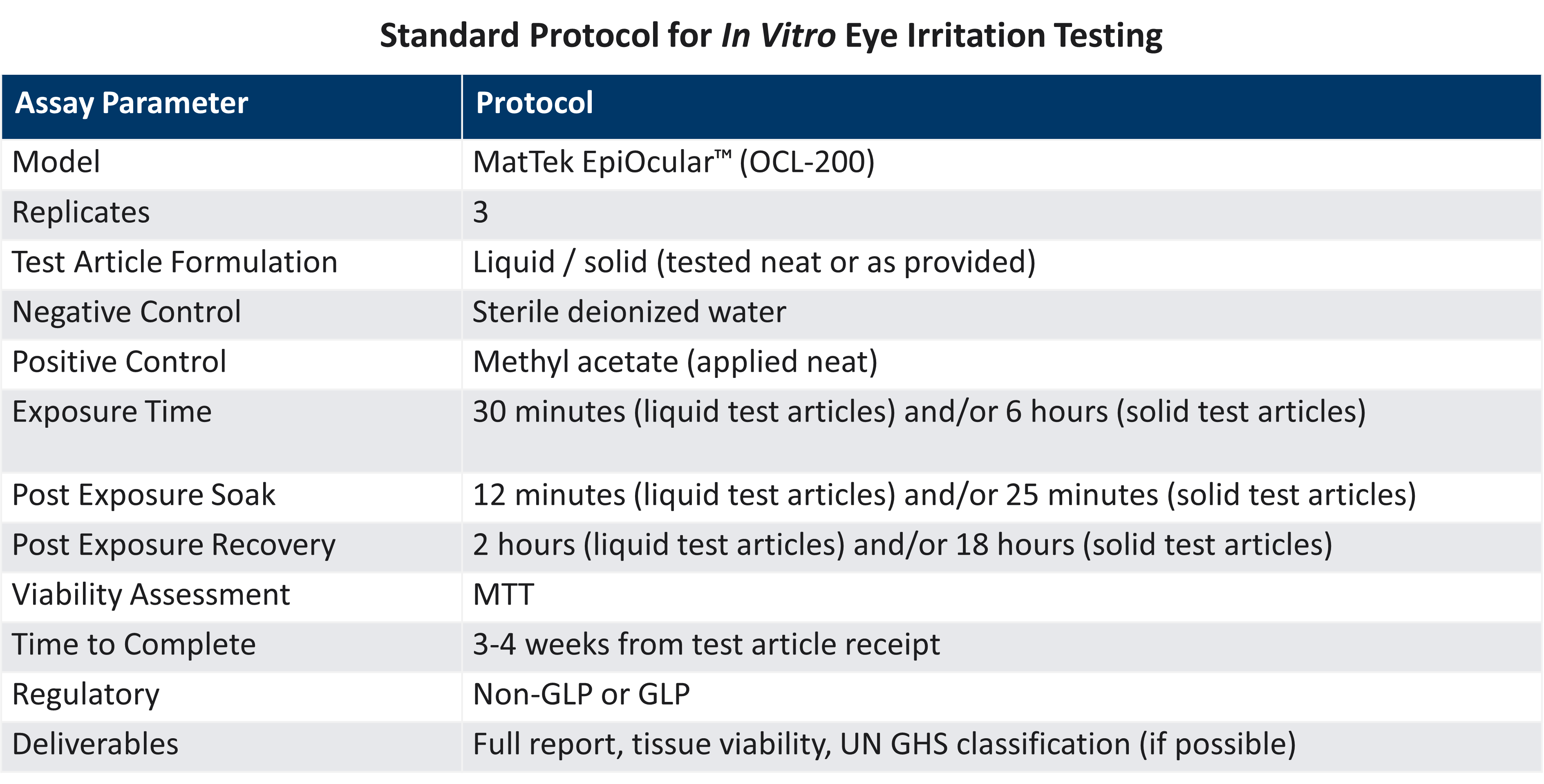
LifeNet Health offers chemical testing services with the validated EpiOcular™ Eye Irritation Test (EIT) for the assessment of potential ocular irritation and/or serious eye damage of the client's test articles, operating in full compliance with the OECD 492 guideline. Test compounds are applied topically to the EpiOcular EIT model using the basic procedures outlined below, and the health of the corneal tissue is assessed by measuring tissue viability immediately following exposure and after
a post-treatment incubation period.
Benefits of Our Services:
- Accurate and reliable data for informed decision-making
- Fast turnaround times to keep your drug development on track
- OECD method-based studies to meet regulatory requirements
- Collaborative approach to ensure the study addresses your research questions
Connect with an expert
