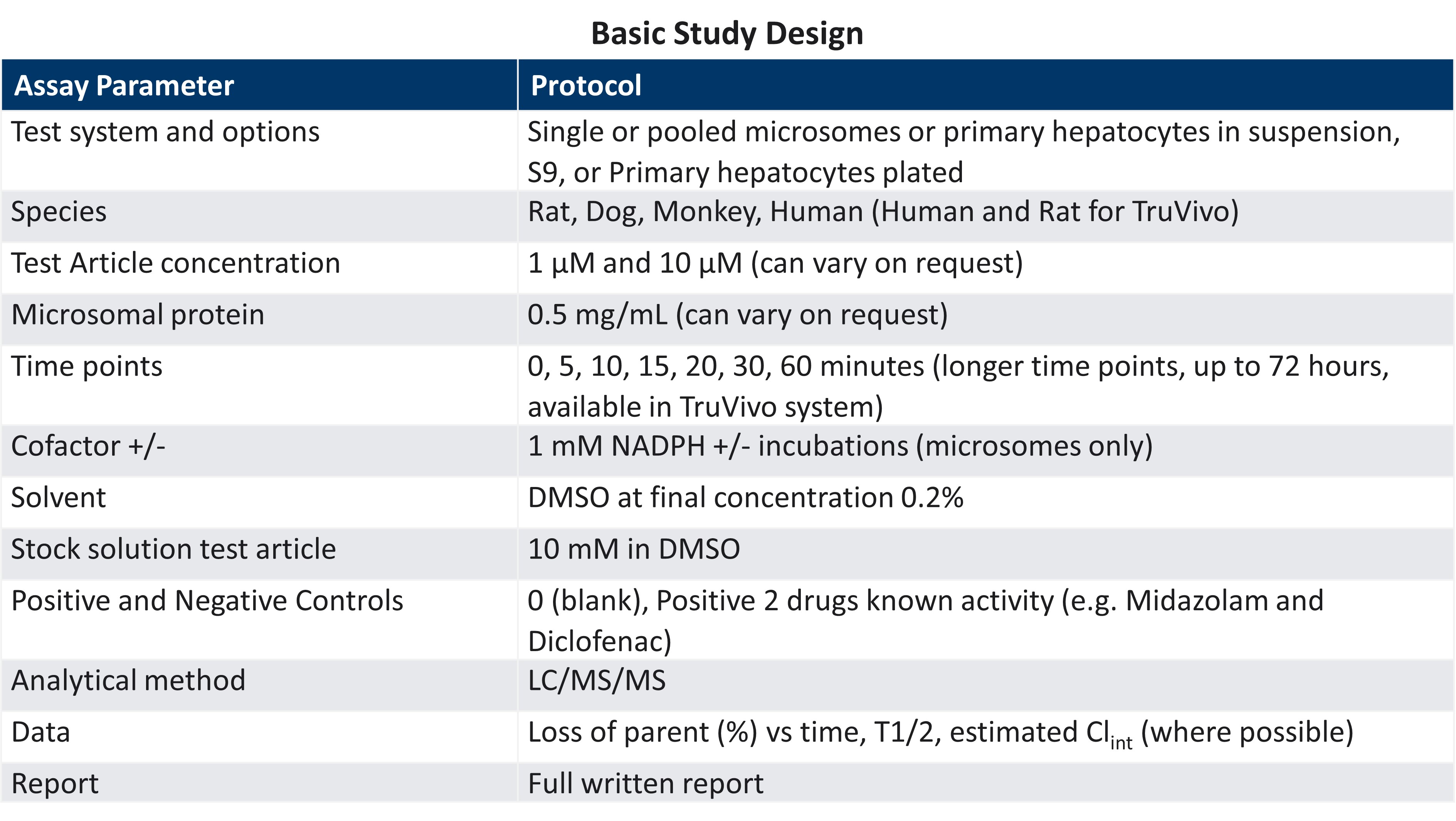
Evaluation of Liver Metabolic Stability
The development of a new drug candidate requires an understanding of its metabolic stability in the liver. Data obtained from these studies can be used to:
- Inform drug design
- Predict intrinsic clearance (Clint)
- Determine half-life (T1/2)
- Support preclinical animal testing and IND submissions
How LifeNet Health LifeSciences Can Help
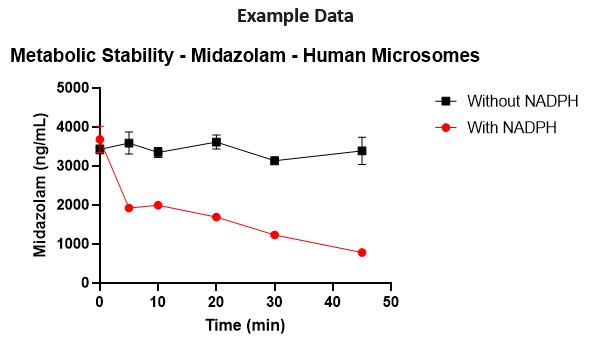
Our TruVivo 2D+ hepatic system can add important information to understanding the metabolic stability of a compound as it possesses key liver properties, including phase II metabolism systems and cell uptake and efflux (transporter) pathways, to test extended exposure times in a more in vivo
With our In Vitro Assay Services team, IND enabling studies testing Clint and T1/2 can be performed with rat and human primary hepatocytes in the TruVivo system, providing a complete and translatable data set for new drug candidates.
Benefits of Our Services:
- Accurate and reliable data for informed decision-making
- Fast turnaround times to keep your drug development on track
- Access to new innovative models and unsurpassed expertise
- Collaborative approach to ensure the study addresses your research questions