Determining Intestinal Absorption In Vitro
For new drugs, it is crucial to determine how much of the administered dose will reach systemic circulation and result in the desired efficacious effect. The primary factors that limit the bioavailability of a drug are:
- Solubility/dissolution of the compounds
- Absorption/crossing of the compound across the intestinal membrane
The Challenge with Testing Intestinal Absorption
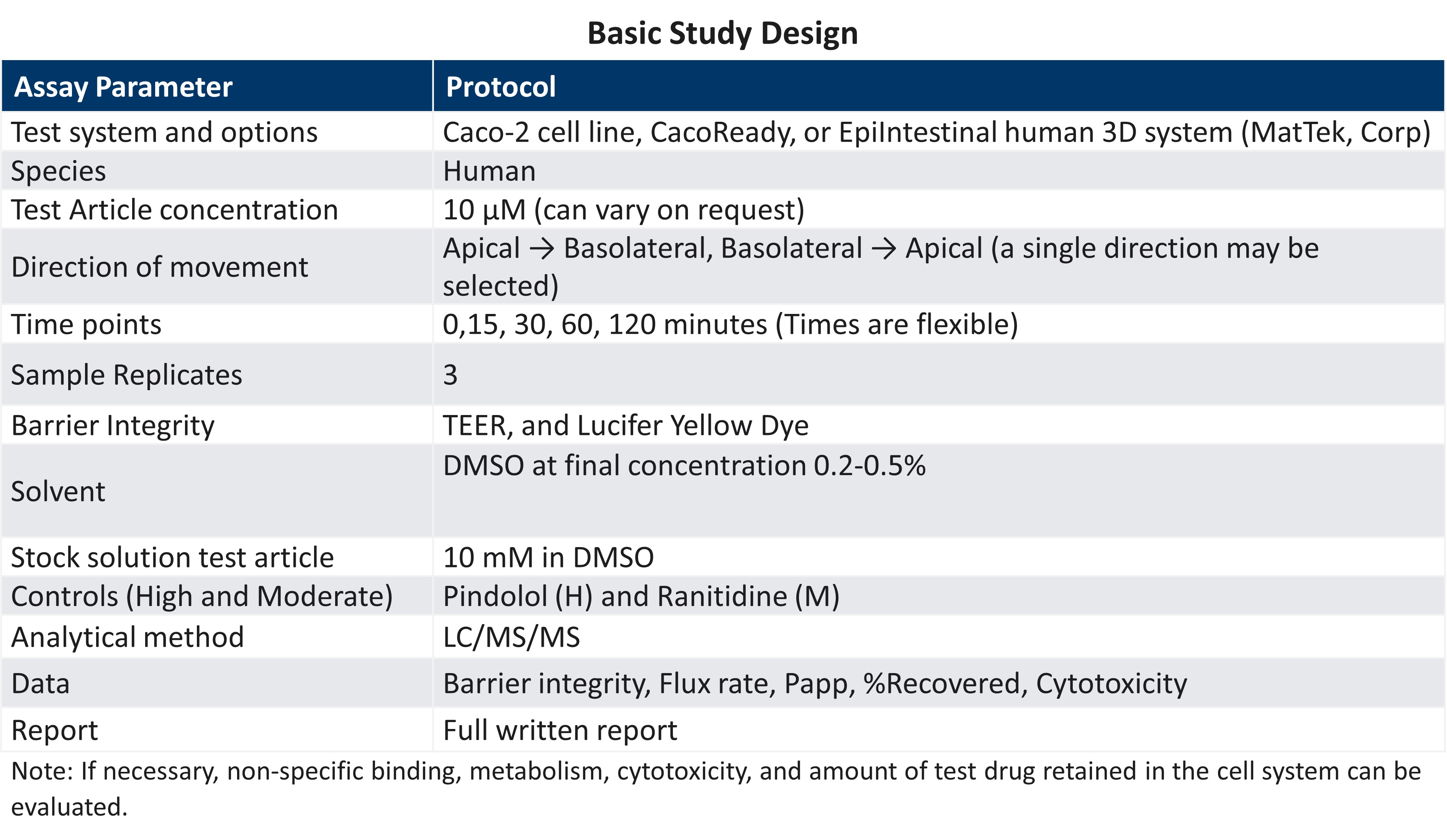
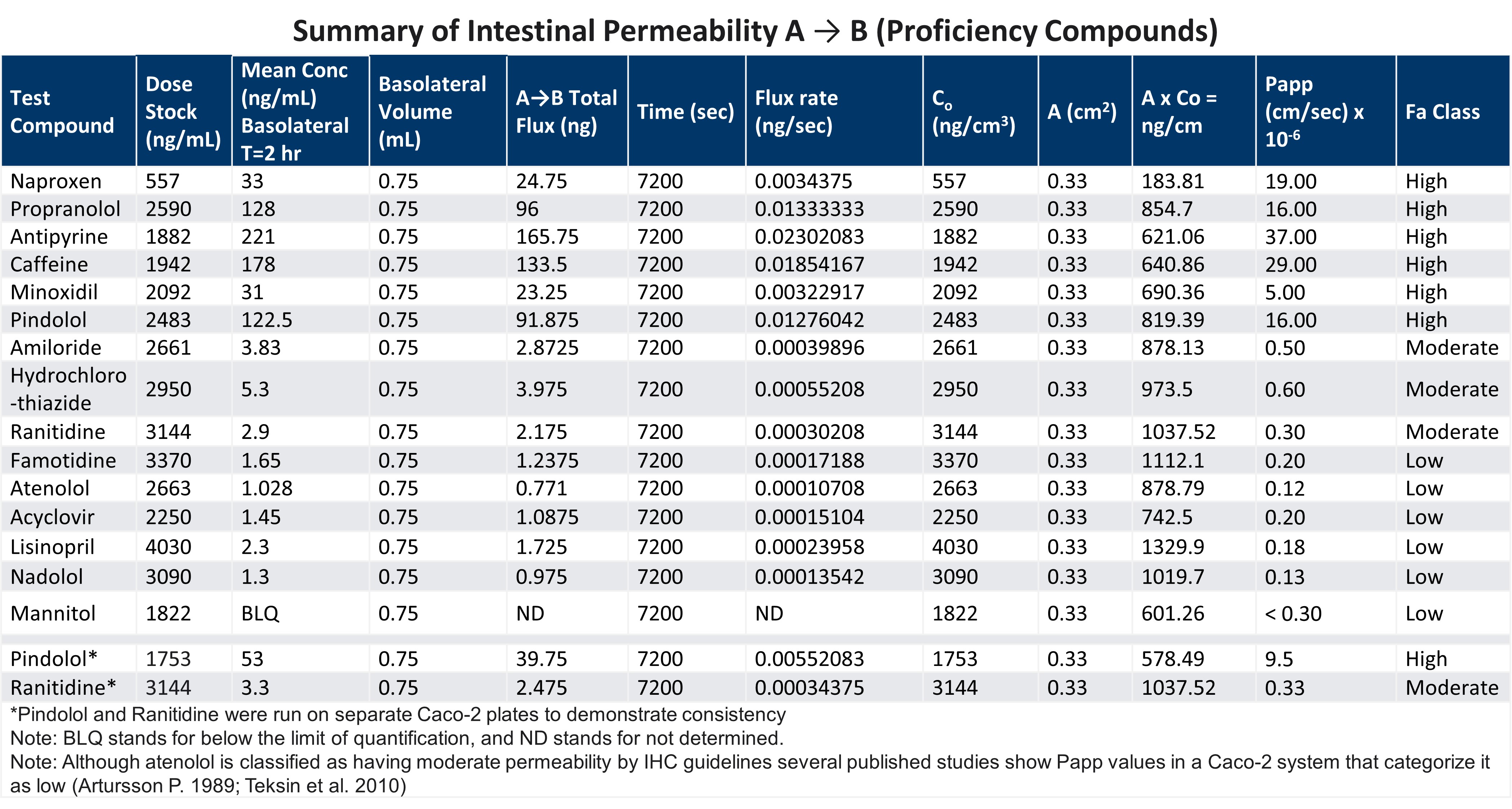
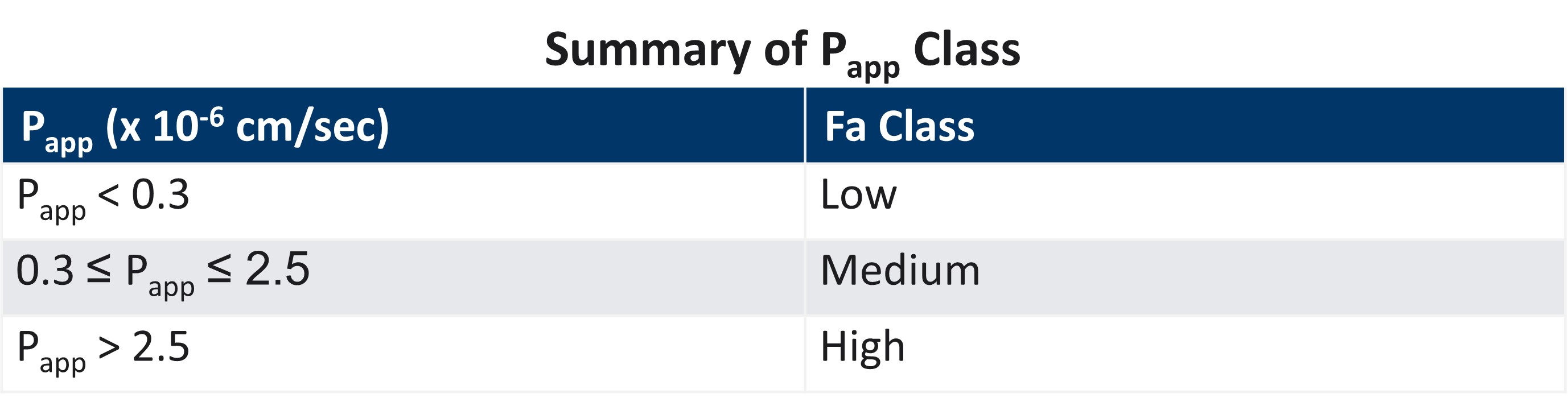
Reproducible high quality intestinal absorption data that can be used to develop pharmacokinetic models for predicting accurate bioavailability (%F) is an essential component of the drug development process. Due to inherent variation within the cell models and interlaboratory variation, accurate and reproducible intestinal absorption (Papp) can be difficult.
The LifeNet Health LifeSciences Solution
LifeNet Health LifeSciences In Vitro Assay Service group is comprised of senior scientists who are highly experienced with the Caco-2 and EpiIntestinal models for understanding intestinal absorption. By using well established culture techniques and validated commercial sources along with inclusion of proficiency compounds, the LifeNet Health team can be sure that the system of the choice is functioning properly, and that the data obtained is accurate.
When these data are combined with liver metabolic stability data, which is also provided by LifeNet Health, the data becomes even stronger. The combined assays allow for a more accurate prediction of %F and systemic exposure.
Benefits of Our Services:
- Accurate and reliable data for informed decision-making
- Fast turnaround times to keep your drug development on track
- Access to new innovative models and unsurpassed expertise
- Collaborative approach to ensure the study addresses your research questions