K.K. Wolf1, J. Pregenzer2, T. Stone1, J.M. McKim2, E.L. LeCluyse1
1LifeNet Health LifeSciences, Research Triangle Park, NC; 2LifeNet Health LifeSciences, Kalamazoo, MI
Abstract
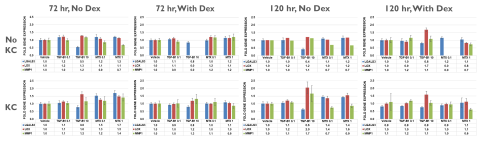
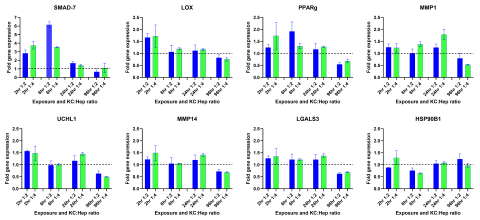
Liver fibrosis is a result of chronic hepatic injury due to various conditions (e.g., viral infection, metabolic disease), as well as drug and chemical exposure. Mechanisms underlying this disease are complex and involve multiple cell types. In vivo animal models have been the gold standard for studying liver fibrosis, but do not fully mimic the immunogenic and metabolic complexities in humans. Most in vitro culture systems, including the organ-on-a-chip system and liver organoids, do not allow for high-throughput screening of potential compound-induced fibrogenic responses in a cost-effective or physiologically-relevant manner. The purpose of this study was to develop an in vitro strategy using TruVivo™, a novel all-human 2D+ hepatic system, with Kupffer cells (KCs) to assess initial fibrogenic responses following compound treatment. The TruVivo system, consisting of adult primary human hepatocytes (PHHs), endothelial cells and stromal cells, was cultured with and without primary KCs, at a ratio of 1 KC:4 PHH or 1 KC:2 PHH, in a glucocorticoid-containing or -free media. Following exposure to methotrexate (MTX; 0.1 and 1 μM) or TGF-β1 (0.1 and 10 ng/mL), known fibrogenic compounds, the expression of various genes known to have a role in fibrogenic responses (e.g., LGAL3, LOX, MMP1) was determined. Cell health was assessed by urea production and LDH release in the medium. All TruVivo cultures (± KCs) remained healthy over the initial culture period. KC presence was confirmed with CD-68 staining. Following compound exposure (up to 120 hr), only those cultures exposed to 10 ng/mL TGF-β1 exhibited signs of cell stress with changes in hepatocellular morphology, urea production, and LDH release. The strongest gene responses following MTX and TGF-β1 exposure occurred with glucocorticoid-free media. In addition, compound, concentration, and time-dependent fibrogenic response gene changes were observed. MTX, at both concentrations, in the presence of KCs, increased LGALS3 at 72 hr (≥1.5-fold) with a slight decline at 120 hr. LOX increased at 72 hr and remained so at 120 hr (≥1.5-fold) with 1 μM MTX, while MMP1 slightly increased (1.4-fold) at 72 hr only. In contrast, TGF-β1, at 10 ng/mL, increased MMP1 in a time-dependent manner in the presence of KCs. Other genes (e.g., MMP14, PPARγ) responded in the opposite manner, with increased expression at earlier time points followed by a decline over exposure time. TGF-β1, at 0.1 ng/mL, did not affect gene expression. In conclusion, the TruVivo system with KCs in a modified medium represents a promising high-throughput in vitro platform that can be used to screen for potential compound-induced fibrogenic responses.
Introduction
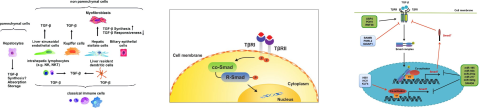
TGF-β synthesis in liver occurs primarily in the non-parenchymal cells (NPCs). Activation of TGF-β receptors (TGFbR) induces signaling via formation of Smad complexes that are translocated to the nucleus where they act as transcription factors. The robustness of TGF-β signaling is controlled by a balance between TGFbR activation and a negative feedback loop via the Smad 7 signaling pathway.1,2
Materials & Methods
TruVivo System3,4
- Tricultures were created by first seeding feeder cells of stromal and endothelial origin in serum-containing TruVivo Plating Medium (TCPM) in collagen I-coated plates (24-well BioCoat® [Corning, Corning, NY] or 96-well [Life Technologies, Carlsbad, CA]); primary human hepatocytes (PHHs; 0.3 or 0.6 x 106 cells/mL, 24- or 96-well culture format, respectively) were added 1-2 hr later. Starting at 4 hr post-hepatocyte plating, the cultures were switched to fresh TruVivo Culture Medium (TCCM) with or without Dexamethasone (Dex; 0.1 μM).
- Kupffer cells (KCs) were added to the tricultures, at a ratio of either 1 KC:4 PHHs or 1 KC:2 PHHs, in the corresponding TCCM ± Dex 24 hr post-hepatocyte plating. Media changes (50%) occurred daily until the start of compound treatment.

Treatments and Assays
- Compound Treatment: Tricultures were exposed to fibrogenic response reference compounds [Methotrexate (MTX; 0.1 or 1 μM; Sigma-Aldrich, St. Louis, MO), TGF-β1 (0.1 or 10 ng/mL; Miltenyi Biotec, Gaithersburg, MD)] or vehicle [DMSO; 0.1% (v/v)] in the corresponding TCCM ± Dex for up to 120 hr starting at Day 5 of culture. No media changes occurred following the addition of compound.
- Cell Viability/Health: LDH release (Promega, Madison, WI) and urea levels (Stanbio Laboratory, Boerne, TX) were measured in media samples collected at the various time points following compound addition.
- Gene Expression Analysis: RNA was isolated from samples using Qiagen RNeasy mini kits with DNase treatment (Qiagen, Germantown, MD). Reverse transcription was carried out using High-Capacity cDNA Reverse Transcription kits (Applied Biosystems/Life Technologies, Grand Island, NY). qPCR was performed using TaqMan™ Universal PCR Master Mix and TaqMan™ Gene Expression Assays for the genes of interest (Applied Biosystems/Life Technologies).
- IHC: Tricultures were established in 96-well plates as described above. At 96 hr post-plating, the cultures were fixed and stained for CD68, HNF-4α, and DAPI, as described previously.3,4
Results
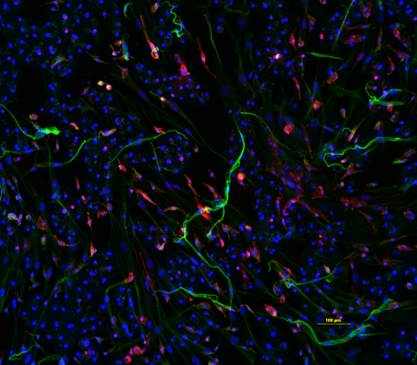
Conclusions
- The TruVivo + KCs model system successfully captured fibrogenic compound effects on target genes. Both the presence of KCs and the absence of DEX in the medium had a positive impact on target gene expression.
- A time- and titer-dependent expression of inflammatory and fibrogenic response genes was observed, possibly through a Smad-mediated feedback loop, indicating that exposure time is important for detecting early vs. progressive effects.
- KC titers may be more important at shorter exposure times and further exploration is warranted.
- Cytotoxicity based on LDH was low, and hepatocytes and feeder cells appeared healthy; urea production was consistent after extended exposure to TGFb, but reduced production may indicate some stress at 96 hr (data not shown).
- The results suggest that the TruVivo + KCs system represents a promising new tool to assess immune-mediated toxicities.
References/Acknowledgements
- Schon HT, Weiskirchen R. Immunomodulatory Effects of Transforming Growth Factor-β in the Liver. Hepatobiliary Surg Nutr. 2014 Dec;3(6):386-406.
- Tu S, Huang W, Huang C, Luo Z, Yan X. Contextual Regulation of TFG-β Signaling in Liver Cancer. Cells. 2019 Oct 22;8(10):1235.
- Weaver JR, Odanga JJ, Wolf KK, Piekos S, Biven M, Taub M, LaRocca J, Thomas C, Byer-Alcorace A, Chen J, Lee JB, LeCluyse EL. The Morphology, Functionality, and Longevity of a Novel All Human Hepatic Cell-based Tri-culture System. Toxicol In Vitro. 2023 Feb;86:105504.
- Odanga JJ, Gianulis E, Whaley L, LeCluyse EL, Presnell S, Weaver JR. An All-Human Hepatic Culture System for Drug Development Applications. J Vic Exp. 2023 Oct 20;(200).
