J.M. McKim1, D. Austin1, R. Sprando2, S. Hermansky2, W. Mattes2, S. Fitzpatrick2
1LifeNet Health LifeSciences, Kalamazoo, MI, 2US FDA/SFSAN, College Park, MD
Abstract
Background and Purpose: Usnic acid (UA) is a secondary metabolite obtained from lichens from the genus Usnea. UA has been reported to have a broad range of therapeutic uses that includes anti-inflammatory, antibacterial, and antiviral properties. In addition, UA is used in perfumes and cosmetics. Along with the reported beneficial effects, oral consumption of UA has also been associated with allergic reactions and with severe liver toxicity. It is therefore important to have new alternative methods that can be used to measure bioavailability, distribution, and organ-specific toxicity, metabolites, and kinetics in an in vitro system that is relevant to humans. This novel approach is needed to meet the demands of next generation risk assessment (NGRA). The primary aim of this study was to evaluate a new meso-scale microphysiology system that integrates multiple organs via a simulated blood flow system.
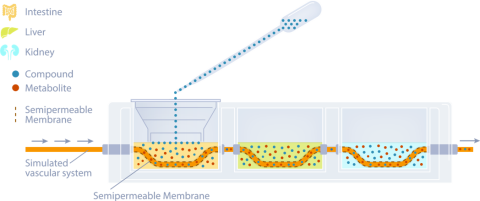
Methods: This in vitro MPS platform consisted of a three-compartment circuit (intestine, liver, and kidney). The human 3D EpiIntestinalTM tissue was obtained from MatTek Corp.; the primary human hepatocytes from LifeNet Health were used for the liver; and the kidney was modeled with a normal human renal proximal tubule cell line (HK-2). Each organ compartment was isolated, and there was no fluid exchange between organ compartments. This allowed each organ model to be cultured under its own optimal conditions. Communication between organs was through a simulated blood flow system that incorporates a semipermeable membrane inside each organ compartment. This allows the test chemicals to move by osmotic diffusion into and out of the blood flow and organ compartments. Salts and other growth medium components are osmotically balanced. Flow was accomplished with a precision micro syringe pump (5 µL/min) and the simulated blood consisted of buffered saline (pH 7.4) with human serum albumin (0.1-0.4%). UA was applied to the apical surface of the intestine chamber (2, 20, and 60 µg). Samples (50 µL) were collected from each compartment at 0 hr, 5 min, 15 min, 30 min, 60 min, 1.5 hr, 2 hr, 4 hr, 8 hr, 24 hr, 48 hr, and 72 hr. These samples were analyzed by LC/MS/MS for UA, and kinetic concentration versus time curves were developed for each compartment. Following the 72-hour collection, the tissues were harvested and used to measure cytotoxicity.
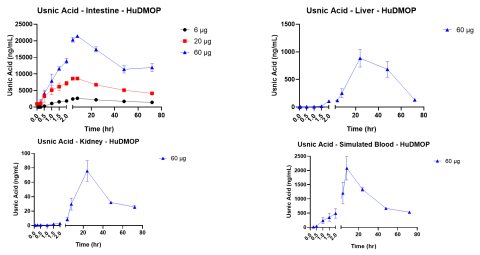
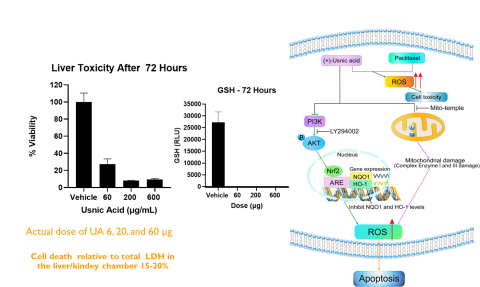
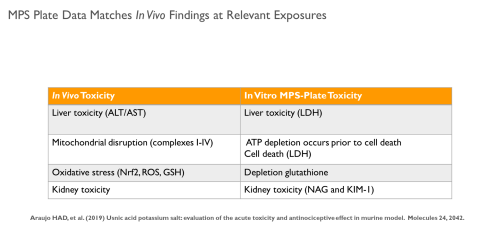
Results: Intestinal exposure to UA reduced a marker of mitochondrial function (MTT) by 60-80% in a concentration dependent manner; however, cell death measured by lactate dehydrogenase leakage (LDH) was only 15-20%. In the liver, ATP levels were significantly reduced in a concentration dependent manner. Reduced glutathione (GSH) was completely depleted at all concentrations. LDH leakage was observed with cell death estimated at 20%. In the kidney, a similar cytotoxic profile was observed.
Conclusions: These data indicate that UA targets the mitochondria and produces oxidative stress in cells, with the most significant effects in liver. These findings are consistent with in vivo data reported by other laboratories. By combining kinetic data, physical chemical properties, and cytotoxicity data, it will be possible to develop PBPK models for predicting exposure and identifying hazard for NGRA.
Introduction
Usnic acid (UA) is an abundant secondary metabolite of lichens and the most common dibenzofuran derivative (Figure 1). UA has been reported to have therapeutic effects such as anti-inflammatory, anti-tumor, anti-bacterial and anti-viral (Ramos and Almeida 2010; Vijayakumar et al. 2000; Backorova et al., 2012). In recent years, UA has been linked to severe liver toxicity including necrosis, hepatitis and liver failure (Brown, AC 2017; Felix et al. 2009). The US Food and Drug Administration (FDA) posted warnings about using one dietary supplements product containing UA, among other ingredients, in 2001. The association of UA with liver toxicity has become an area of interest for biomedical research. Many compounds require further study in order to evaluate potential risk for human consumption. The use of animals for toxicity testing is time consuming and expensive. As a result, research groups are highly interested in the possibility that integrated organ systems that could provide rapid hazard assessment and provide data regarding mechanism(s) of toxicity and organ specificity. Therefore, the purpose of this study was to evaluate UA in a novel MPS platform that links human intestine, liver, and kidney by a simulated blood system.
Materials & Methods
The intestine was modeled using EpiIntestinal by MatTek Corp.. The liver compartment was modeled with well-characterized primary human hepatocytes from LifeNet Health and the kidney chamber was normal human renal proximal tubule cells (HK-2) from ATCC. The three organ systems were completely isolated and cultured with media specific for each tissues optimal growth. Each tissue chamber held 2.5 mL of medium. Communication between compartments was achieved by a simulated vascular system driven by a micro syringe pump at 5 µL/min. The section of vascular tubing inside each compartment was a semipermeable membrane (Mw cut off 30K da.). The movement of test material from one compartment to another is by osmotic movement of the test material (UA) and any metabolites into and out of the simulated vasculature system. The fluid being pumped through this tubing can be modified as needed, but typically consisted of phosphate buffered saline (PBS pH 7.4) and 0.1% human serum albumin (Figure 2).
Prior to beginning the experiment, UA non-specific binding, and an in vitro maximally tolerated dosage (MTD) were determined. Dose selection was based on in vivo pharmacokinetic data reporting the Cmax for UA to be approximately 60 µg/mL following an IV administration. (Venkataramana and Krishna (1993)).
The kinetic experiments were started by adding 100 µL of the UA stock solutions to the apical side of the EpiIntestinal model. Samples from all compartments were collected at the indicated times and the amount of UA determined by LC/MS/MS. Following the 72 hr exposure, the tissues were collected and analyzed for cell health. Assays included the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for the intestine tissue, adenosine triphosphate (ATP) and glutathione (GSH) for the liver tissue, and N-acetyl-β-d-glucosaminidase (NAG) for the kidney.
Results
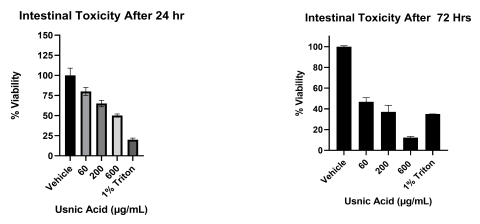

Conclusions
- The integrated organ MPS platform produced kinetic curves for UA that were dose dependent.
- At relevant in vitro doses, UA had significant cytotoxic effects in the liver.
- The primary mechanism for cytotoxicity was mitochondrial disruption.
- These findings are consistent with those reported in the literature.
- This MPS platform is applicable to rapid response testing and hazard identification.
References/Acknowledgements
Backorova, M.; Jendzelovsky, R.; Kello, M.; Backor, M.; Mikes, J.; Fedorocko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. Vitr. 2012, 26, 462–468
Brown, A.C. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem. Toxicol. 2017, 107, 472–501
Felix, S.; Sara, D.; Eleonora, P.; Katja, B.; Beat, A.; Stephen, L.L. Severe hepatotoxicity following ingestion of Herbalife® nutritional supplements contaminated with Bacillus subtilis. J. Hepatol. 2009, 50, 111–117
Ramos, D.F.; Almeida, D.S.P. Antimycobacterial activity of usnic acid against resistant and susceptible strains of Mycobacterium tuberculosis and non-tuberculous mycobacteria. Pharm. Biol. 2010, 48, 260–263.
Venkataramana, D., and Krishna, DR (1993) Pharmacokinetics of Usnic acid in rabbits after intravenous administration. Europ J drug Metab and Pharmacok 18, 161-163.
Vijayakumar, C.S.; Viswanathan, S.; Reddy, M.K.; Parvathavarthini, S.; Kundu, A.B.; Sukumar, E. Anti-inflammatory activity of (+)-usnic acid. Fitoterapia 2000, 71, 564–566
