Hlaing H. Maw, Ting Wang, Klairynne Raymond, Alexander Byer-Alcorace, Stephanie Piekos, Tom S. Chan, and Mitchell E. Taub
Boehringer Ingelheim Pharmaceuticals Inc., Drug Metabolism and Pharmacokinetics Department, Ridgefield, CT, USA
Abstract
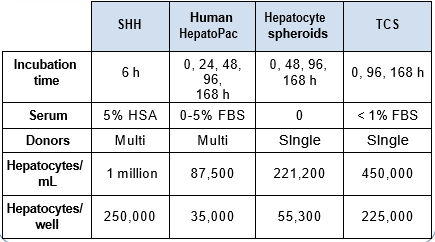
Isolated human hepatocytes in suspension (SHH) are routinely used as an in vitro tool to investigate hepatic metabolism. However, the use of SHH is limited by a relatively short incubation time (≤ 6 h), which can be inadequate to form metabolites for slowly metabolized compounds. Long-term primary human hepatocyte culture models such as HepatoPac, spheroids, and most recently the Triculture system (TCS), have been developed to address this challenge. The objective of this study is to compare the performance of these three models and SHH as in vitro tools to generate Phase I and II metabolites observed in vivo. BI 425809, BI-A, and TAK-041 were investigated due to their absent or low metabolism in SHH and their diverse circulating metabolites in humans. When BI 425809 was incubated with HepatoPac, spheroids, or TCS, 8 out of 11 circulating metabolites were identified. One unique in vivo pathway was amide hydrolysis of M526. Both M232 and M312, formed via hydrolysis of the amide bond in M526, were detected in spheroids and TCS, but not in SHH or HepatoPac. In human plasma, 2 oxidative and 1 oxidative-glucuronide metabolites of BI-A were identified. Interestingly, the two-step oxidative- glucuronide metabolite, which was not observed in SHH, was detected in incubations with all three long-term culture models. TAK-041 undergoes multistep and sequential downstream metabolism in humans, but there was no metabolite formed in SHH1. Notably, all three long-term culture models can produce sequential in vivo metabolites such as cysteine S-conjugate and glutathione adduct-derived thiol metabolites. Overall, the three long-term culture models investigated generated clinically relevant metabolites at measurable levels. All three systems generated metabolites more extensively than SHH, which is relevant for slowly metabolized compounds, and thus are suitable tools to predict in vivo metabolism.
Methods
Results
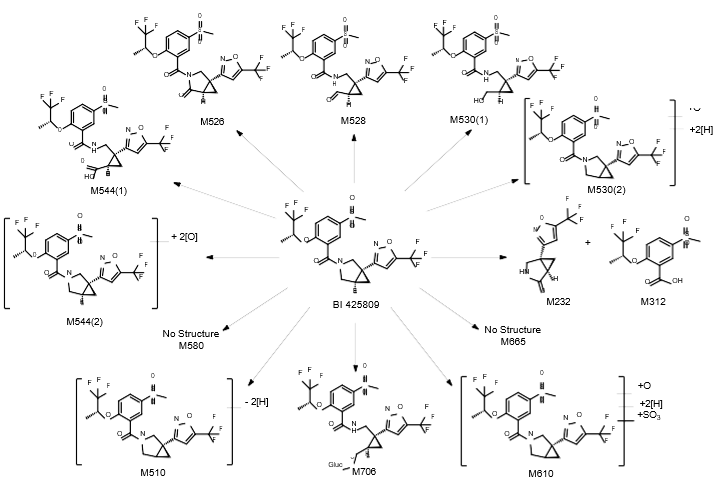
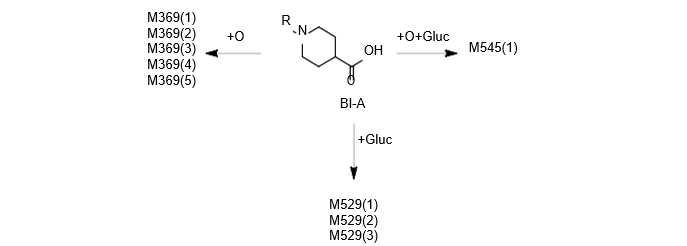
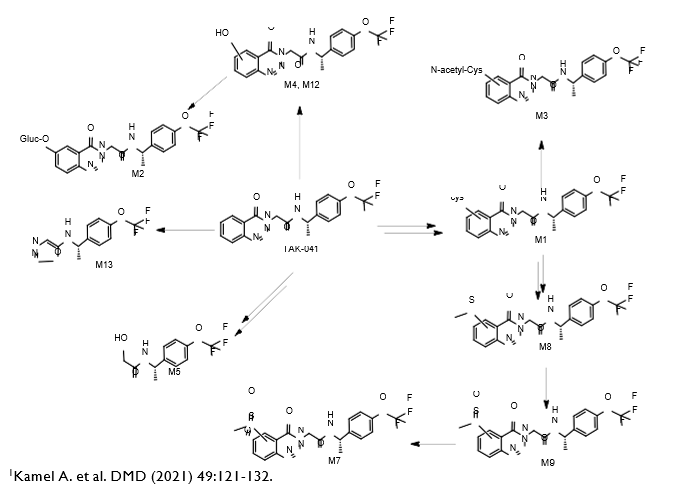
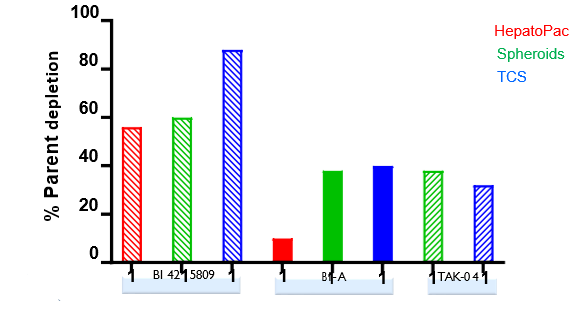
Table 1. BI 425809 and its metabolites identified in human plasma and hepatocytes.
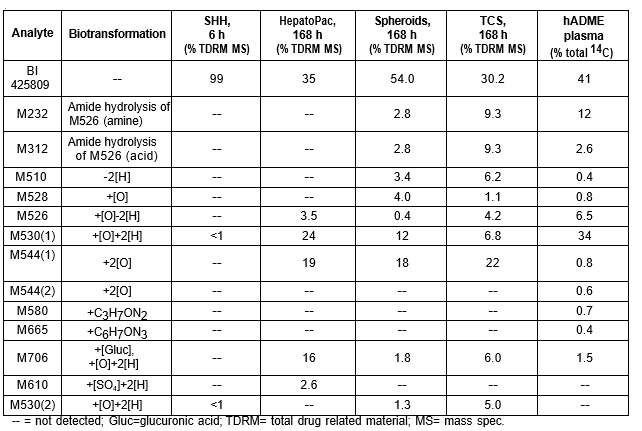
Table 2. BI-A and its metabolites identified in human plasma and hepatocytes.
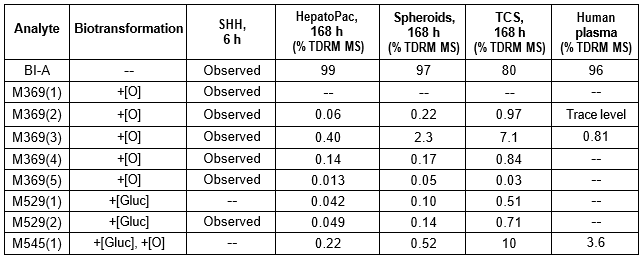
Table 3. TAK-041 and its metabolites identified in human plasma and hepatocytes.
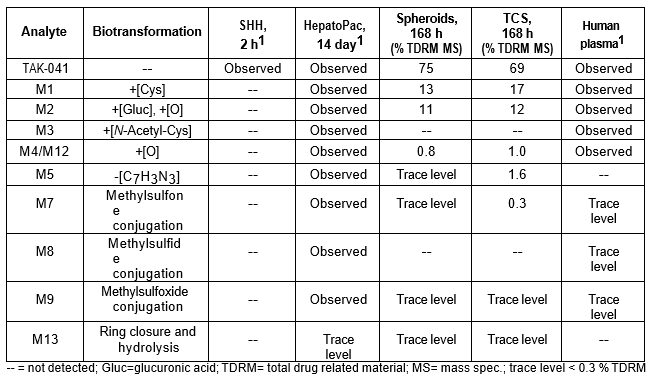
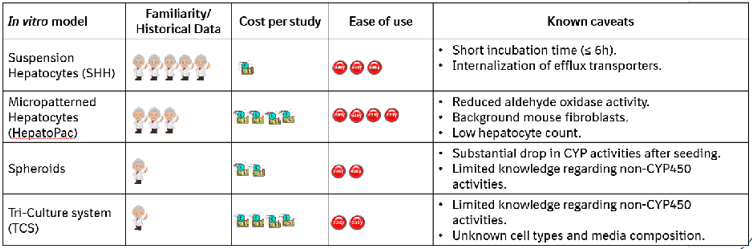
Table 4. Comparison of in vitro models investigated regarding familiarity, cost, ease of use, and caveats.
Conclusions
- Three long-term primary human hepatocyte culture models:
- Generally, form more metabolites than incubations with SHH.
- Can predict in vivo metabolism of slowly and extensively metabolized compounds.
- May be suitable to biosynthesize in vivo metabolites.
- Single donor hepatocyte systems (spheroids and TCS) can generate relevant in vivo metabolites as in the multi donor system (HepatoPac).
- Ease of use, potential limitations, and cost effectiveness need to be carefully considered (Table 4).
- For certain studies, SHH may be adequate, while a long-term hepatocyte culture model may be more suitable for other types of studies.
