Alexander Byer-Alcorace1, Cody Thomas1, Stephanie Piekos1, Jessica Weaver2, Justin J. Odanga2, Kristina K. Wolf3, Jingsong Chen2, Jung Bok Lee2, Edward L. LeCluyse3, and Mitchell E. Taub1
1Nonclinical DMPK, Boehringer Ingelheim Pharmaceuticals, Inc., 900 Ridgebury Road, Ridgefield, CT 06877; 2Institute of Regenerative Medicine, LifeNet Health, 1864 Concert Drive, Virginia Beach, VA 23453; 3Research and Development, LifeSciences, LifeNet Health, 6 Davis Drive, Research Triangle Park, NC, 27709
Introduction
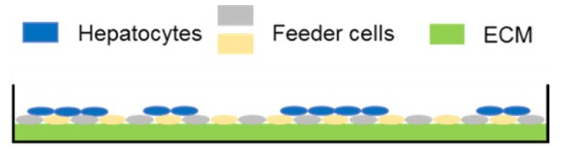
Predicting human hepatic clearance (CLhepatic) is often challenging in early drug development, particularly when low-clearance compounds are involved. To assess the metabolic stability of such drugs, suspended primary human hepatocytes (PHH) are commonly used; however, their ability to accurately predict in vivo CL, particularly of slowly cleared drugs, can be limited by short incubation times and rapid loss of enzymatic activity. To mitigate these issues, long-term in vitro models, like the Triculture System (TCS) developed by LifeNet Health, have been implemented in an effort to improve the characterization of hepatic metabolism and clearance in vitro by lengthening the amount of time hepatocytes can be cultured successfully. The TCS is an all-human cell-based in vitro model comprised of PHH and two different types of primary feeder cells (FC) that are plated together, shown in (Figure 1), on either a 24-well or 96-well collagen-I coated plate. In this study, the TCS (in both 24- and 96-well formats) was assessed for its functionality and its ability to predict human CLhepatic over the course of a 7- day incubation with no media change.
Experimental Methods

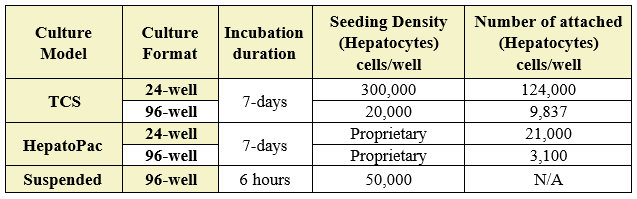
Table 1. Incubation conditions. Number of cells seeded, cells attached, and incubation duration in both 24- and 96-well formats. (N/A; not applicable as cells were in suspension).
Comparison of basic cellular function and CLhepatic in both 24- and 96-well formats:
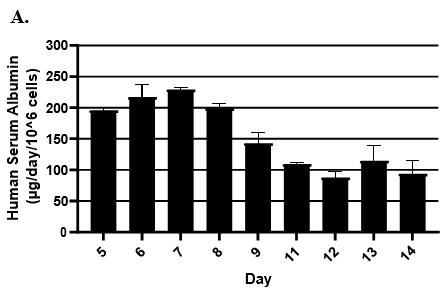
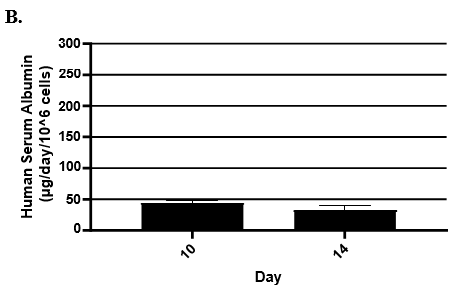
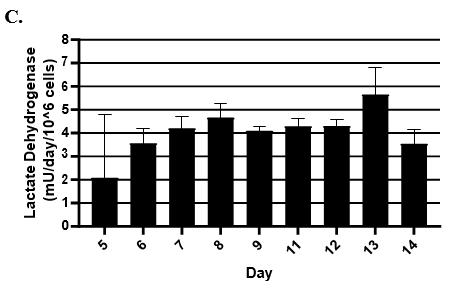
- Hepatic health was evaluated by measuring albumin and lactate dehydrogenase (LDH) at each timepoint normalized to 24h for up to 9-days in culture with no media change.
- Albumin secretion was assessed using the Abcam Human Albumin SimpleStep 90min ELISA kit.
- LDH release was measured using Promega's LDH-Glo Cytotoxicity kit.
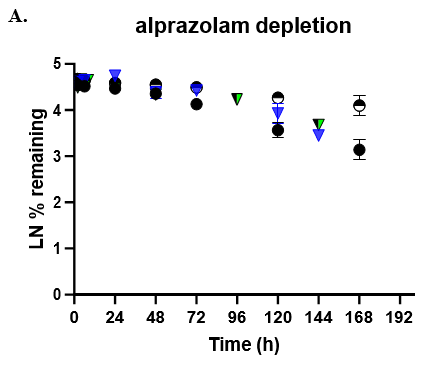
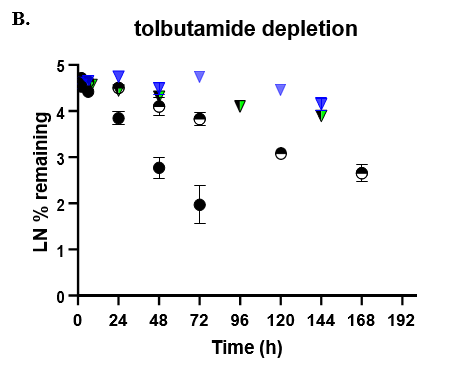
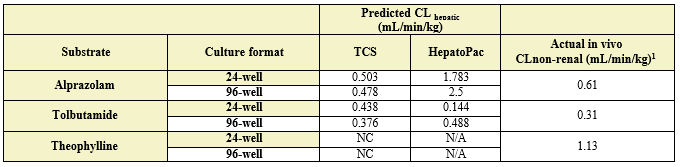
- TCS CLhepatic was assessed by administering test compount and monitoring parent depletion over a 7-day incubation with no media change.
- Predicted CLhepatic was compared to well established models such as HepatoPac (multidonor lot) and suspended hepatocytes (TCS donor lot).
- Depletion was quantitated by LC-MS/MS.
Table 2. Incubation substrates and clearance pathways.

Results
![]()
![]()
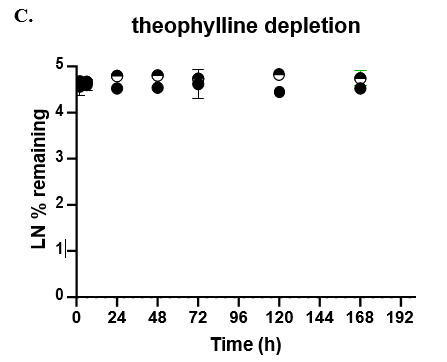

Table 3. Comparison of predicted in vitro CLhepatic values for alprazolam, tolbutamide, and theophylline to actual in vivo non-renal clearance. Appropriate scaling parameters were applied to determine the predicted CLhepatic and all formats were normalized to 106 cells. (N/A) not applicable as it was not assessed; (NC) not calculated, no detectable depletion.
Conclusions
Hepatocytes in both the 24-well and 96-well formats appeared healthy morphologically and functionally demonstrated high levels of albumin secretion and relatively low levels of LDH release with no media change for up to 7 days when compared to values observed with daily media changes.
The TCS predicted CLhepatic that is within 2-fold of actual human in vivo non-renal CL values in both the 24-well and 96-well formats for 2 of 3 compounds tested. Theophylline CLhepatic could not be calculated as no depletion was observed, potentially due to low CYP1A2 activity in this donor. The TCS shows that it performs equally to that of current established hepatocyte systems.
Overall, the TCS can be used to assess and characterize potential drug candidates for their metabolic stability and clearance in vitro while maintaining hepatic function in long duration incubations.
References
Chan TS, Yu H, Moore A, Khetani SR, Tweedie D. Meeting the challenge of predicting hepatic clearance of compounds slowly metabolized by cytochrome P450 using a novel hepatocyte model, HepatoPac. Drug Metab Dispos. 2013 Dec;41(12):2024-32. doi: 10.1124/dmd.113.053397. Epub 2013 Aug 19. Erratum in: Drug Metab Dispos. 2014 Jan;42(1):200. Kehtani, Salman R [corrected to Khetani, Salman R]. Erratum in: Drug Metab Dispos. 2019 Jan;47(1):58-66. Corrected and republished in: Drug Metab Dispos. 2019 Jan;47(1):58-66. PMID: 23959596.
