Angela Murchison, Kristina K. Wolf, Erick K. Breathwaite, Jessica R. Weaver, Michelle L. Treadwell, Valerie Soldatow, JingSongChen, Edward L. LeCluyse, Jung Bok Lee
Abstract
Predicting drug- and chemical-induced hepatotoxicity remains a great challenge. Animal-based testing during compound development too often fails to identify this risk, unable to mimic the role of non-parenchymal cells in response to compound perturbations and disease progression. Conventional in vitro hepatic model systems, such as monocultures of primary human hepatocytes, lack or exhibit the loss of key functions as well as cellular architecture and integrity over time. We have developed an all-human Tri-Culture System, in both 24- and 96-well formats, comprised of primary human hepatocytes with endothelial and stromal cells derived from donated human tissues. Optimal cell densities and ratios were established based on the restoration and maintenance of key self-assembled cellular structures and functions over a 4-week period, measured by the localization of key cell-cell interaction and junctional polarity markers, albumin and urea synthesis, and CYP basal and induced activities. Experiments comparing mono- and tri-cultures of hepatocytes from multiple donors, healthy and diseased, demonstrated that basal albumin and urea synthesis rates and CYP basal and induced activities decreased markedly (>50% decrease of albumin and urea, undetectable levels of CYP basal expression due to cell loss) in monocultures. These cellular and biochemical parameters increased in the tri-cultures over the initial 4 to 7 days, reaching steady-state levels between 7 and 10 days (albumin and urea expression maintained within a 20% range, and CYP fold change of >10). These promising results directly corresponded to hepatocellular remodeling facilitated by ZO-1- and Cx32-mediated cell-cell interactions and cell junction and functional bile canaliculi formation. Regardless of donor background, the presence of non-parenchymal cells sustained and enhanced hepatocyte integrity, polarity and performance over several weeks in culture. Overall, this all-human Tri-Culture System represents an exciting and promising new analytical tool for studying the efficacy and liability of compounds on hepatocellular function of both healthy and diseased human donors.
Introduction
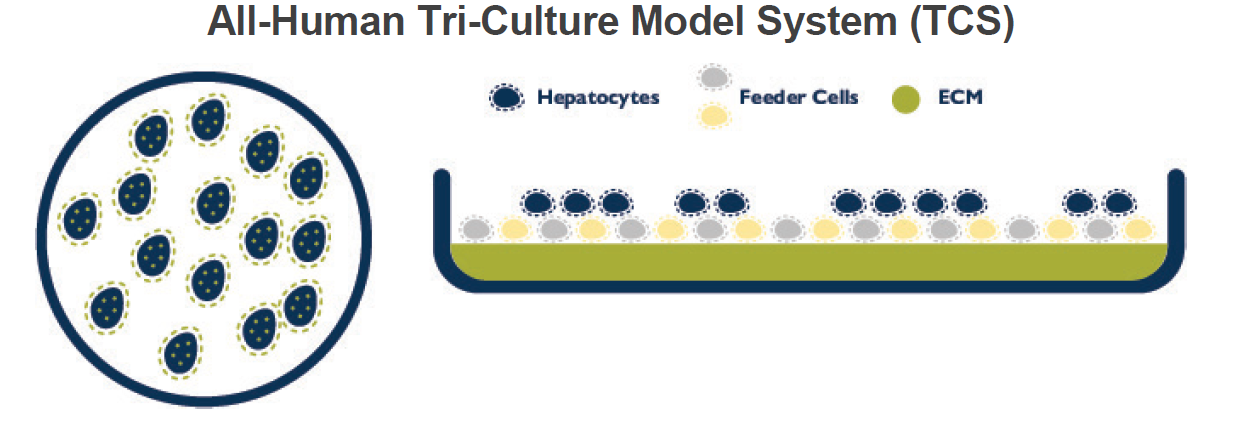
The purpose of these studies was to develop and validate an all-human hepatic cell-based co-culture model system that would sustain hepatocyte phenotypic architecture, basic liver functions and metabolic capacity for prolonged periods of time for both pathological and toxicological investigations. Previous studies had shown enhancements to cellular integrity, response to receptor agonists and metabolic capacity using co-cultures of mouse 3T3 fibroblasts (1-3). However, there is a need for an all-human and donor-matched co-culture system to mimic the intercellular events that occur during the progression of liver injury and disease. In this study, we describe an all-human hepatocellular Tri-Culture System (TCS) that achieves the initial goal of sustaining architectural integrity and basic functions of human hepatocyte batches from multiple donors representing different backgrounds of normal and diseased liver tissue.
Materials and Methods
Hepatocyte Culture Systems
Monoculture samples were prepared with cryopreserved primary human hepatocytes (PHH) at a density of 150,000-250,000 cells/cm2 (batch dependent) on collagen coated 24-well plates. Monoculture samples were overlaid the following morning with Matrigel® (Corning) to create sandwich cultures (SC).
The all-human Tri-Culture System (TCS) was prepared with feeder cells on a collagen-coated 24-well plate and incubated at 37°C for 30 minutes. Cryopreserved PHH were then added at a density of 150,000-250,000 cells/cm2 (batch dependent).
Treatments and Assays
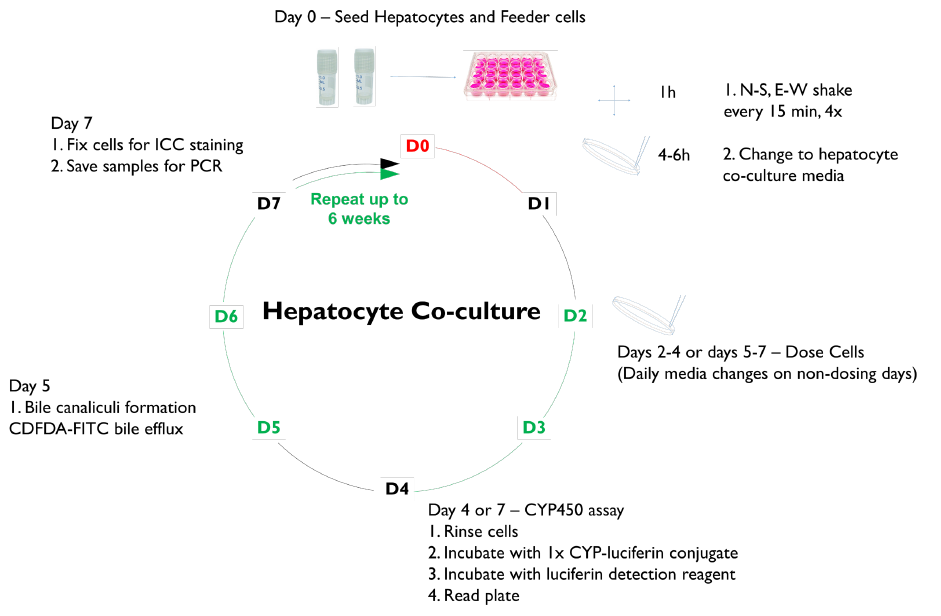
The morphology of each culture model was assessed over a 3–6-week period.
CYP induction response was assessed in SC and TCS hepatocytes after 1 week in culture by treating for 48 hours with the prototype receptor agonists, omeprazole (100 µM), CITCO (100 nM), or rifampicin (25 µM), to induce CYP enzymes 1A2, 2B6, and 3A4, respectively. CYP activities were determined using P450-Glo™ Assays (Promega Corp).
Albumin and urea secretion were measured at select time points in collected media samples following 24 hours of culture using an ELISA kit from Abcam. The localization of PHH clusters, feeder cells, tight junctions, and gap junctions were assessed by immunofluorescent staining. 5(6)-Carboxy-2’,7’-dichlorofluorescein diacetate (CDFDA) staining was used to demonstrate the formation of bile canaliculi and MRP-2 transporter function. NTCP was localized in TCS with an Abcam antibody.
Results



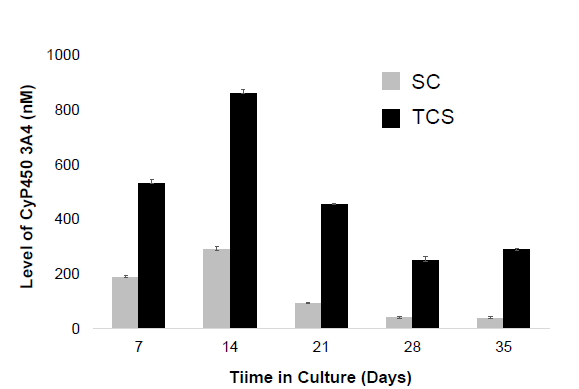
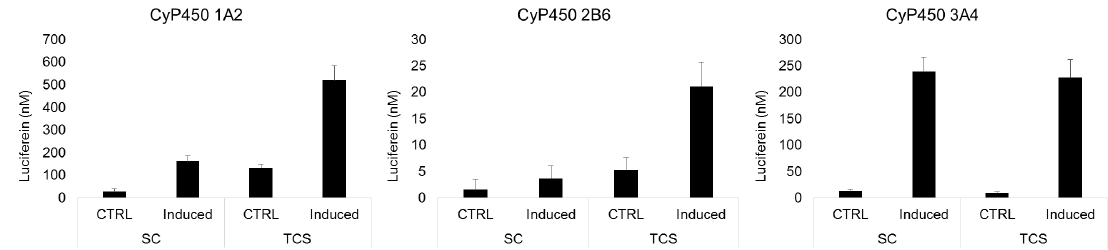
Conclusions
1. The all-human triculture system (TCS) mimics key hepatocellular constituents and multicellular interactions extending and maintaining long-term viability, functionality and metabolic capacity of PHH cultures.
2. The TCS enables prolonged exposures or re-exposures to compounds and their metabolites where needed (e.g., reactive metabolites and conjugates).
3. The TCS is suitable for experiments to determine metabolic profiles, clearance of low-turnover compounds, enzyme induction.
4. The TCS expands the available donor pool to include PHH batches from most healthy and diseased tissues (e.g., NAFLD, NASH and HBV).
References/Acknowledgements
We thank Gary Walters and Danette Fuentes for assistance with tissue recoveries, and Justin Odanga, Dominica Williams, Stacey Hanson, Daniel Shuman, Kristen Hatfield, Jeff Thomas, and Lindsey Whaley for assistance with logistics and poster arrangements.
