Jessica R. Weaver1, Justin J. Odanga1, Kristina K. Wolf2, Tammy Stone2, Stephanie Piekos3, Mitchell Taub3, Cody Thomas3, Alexander Byer-Alcorace3, Jingsong Chen1, Jung Bok Lee1, and Edward L. LeCluyse2
1Institute of Regenerative Medicine, LifeNet Health; 2Research & Development, LifeSciences, LifeNet Health; 3Non-Clinical DMPK, Boehringer Ingelheim Pharmaceuticals, Inc.
Abstract
The use of primary human hepatocytes (PHHs) in preclinical pharmacological and toxicological applications has been restricted in part by the lack of a suitable culture platform that is convenient, supports a multitude of donor lots from healthy and diseased tissues, and maintains the structural and functional properties of the cells over prolonged periods of time. The sandwich culture (SC) and the co-culture systems have addressed some of these issues; however, some limitations remain. To address these limitations, an all-human triculture system has been developed that uses PHHs and primary feeder cells (FCs). Cryopreserved human feeder cells consisting of two types of stromal cells at a 1:1 ratio were thawed and seeded onto a 24-well collagen coated plate. Cryopreserved adult PHHs were then thawed and plated onto the FCs, creating a triculture system (TCS). Morphology and functionality, including albumin (Alb) and urea secretion and cytochrome P450 (CYP) activity, were evaluated over a 3-week culture period. PHHs from multiple adult donor lots in the TCS exhibited a healthy and stable morphology, including multicellular cluster formation, for up to 42 days. Extensive anastomosing networks of bile canaliculi with tight and gap junctions were established during the initial 5 days of culture and remained stable throughout the remainder of the culture period. Albumin and urea production levels were significantly higher in the TCS (30-40 µg/day/106 cells and 55-70 µg/day/106 cells, respectively) over a 3-week period compared to SC monoculture PHHs. Gene expression of Alb, CYP1A2, CYP2B6, and CYP3A4 in the TCS PHHs at day 4 was increased 2-fold on average compared to the PHHs in the SC monoculture. Phase 1 and phase 2 metabolic function (midazolam 1-hydroxylation rates: 10-20 pmol/min/106 cells, 7-ethoxycoumarin glucuronidation rates: 300-600 pmol/min/106 cells) was stable after 5 days for at least 2 weeks. Overall, these results show that the triculture system represents a convenient all-human hepatic culture system that maintains both hepatocellular morphology and metabolic function over several weeks.
Introduction
Existing limitations for finding suitable culture models or systems that mimic the in vivo liver microenvironment include the ability of the hepatocytes to maintain their morphology and functionality over prolonged periods of time.1 Animal models can be used, but there are marked species differences in hepatic metabolic and receptor-signaling pathways.2 Mono- and sandwich culture model systems also have corresponding serious limitations including the short- to mid-term loss of cell polarity and metabolic functions.3 Co-culture and triculture models that currently exist combine hepatocytes with one or more different type of FCs. However, these systems use non-human FCs, primarily mouse or rat origin.4 In this newly developed TCS, two different types of frozen primary human FCs and PHHs are used. The kit component of cryopreserved cells is convenient and has an ease of use for the researcher that allows control over the experimental work flow in a standard plate format. It can be set up in a matter of hours and tested in multiple applications that require a sustained hepatocyte polarity and phenotype.
Materials and Methods

Expression of ZO-1, Connexin-32, Cytokeratin 18 (CK18), Alb, CYP1A2, and Vimentin (VIM) were assessed by immunocytochemistry with antibodies from Thermo Fisher and Abcam respectively. 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain was used to stain nuclei. 5-(and-6)-Carboxy-2’,7’-Dichlorofluorescein Diacetate (5µM) (CDFDA, Thermo Fisher) was used for bile canaliculi staining.
Cells were lysed in RLT buffer (Qiagen). Total RNA was isolated using the RNeasy Mini Kit (Qiagen). cDNA was prepped using the QuantiNova SYBR Green RT-PCR kit (Qiagen). PCR amplification was done on a StepOnePlus RT PCR System (Applied Biosystems) and analyzed with StepOne Software. Data was normalized to GAPDH and analyzed using the 2-∆∆C method.
Supernatant was collected for Alb and urea detection. Alb was measured using an ELISA kit (Abcam), and a colorimetric assay (StanBio Labs) was used to measure urea synthesis.
CYP1A2, CYP2B6, and CYP3A4 activity were measured in PHHs after being induced for 48hrs with Omeprazole (100µM), CITCO (100nM), and Rifampicin (25µM) using a P450-Glo Assay (Promega).
Phase I metabolism was determined by LC MS/MS using Midazolam (15µM) as a probe. Samples were analyzed for concentrations of 1’-hydroxymidazolam (OH-MDZ).
Phase 2 metabolism was determined by LC MS/MS. Cells were exposed to 100µM 7-ethoxycoumarin (7-EC) from Sigma. Samples were collected following a 30(60)-minute incubation period and analyzed for production of 7-hydroxycoumarin (7-HC), 7- hydroxycoumarin glucuronide (7-HCG) and 7-hydroxycoumarin sulfate (7-HCS), by LC-MS/MS.
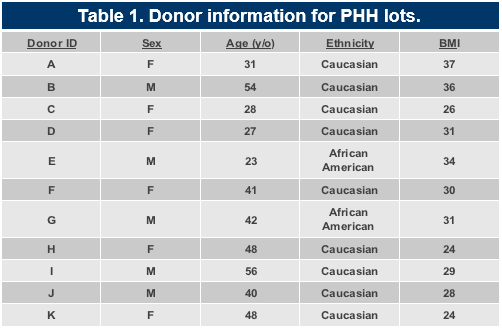
Results
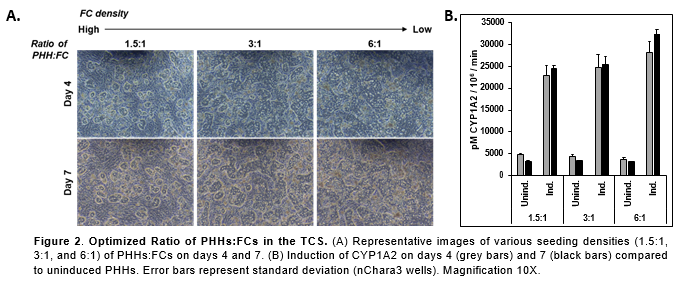
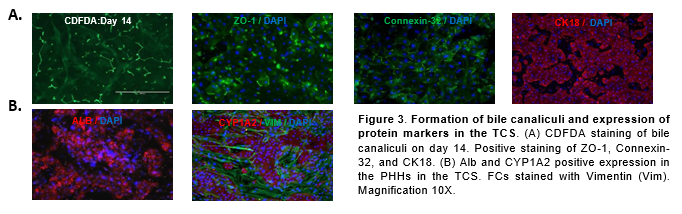
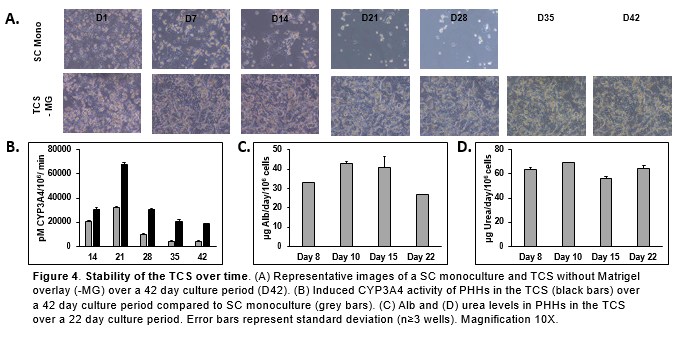
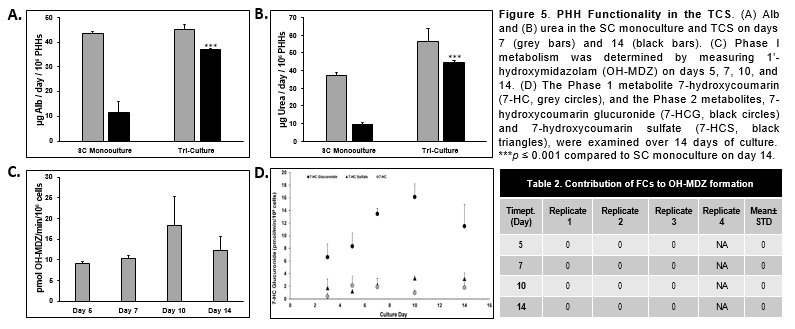
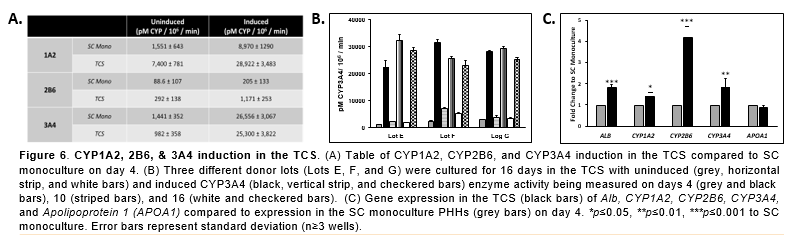
Conclusions
- PHHs in the TCS are able to maintain hepatocyte morphology for 42 days in vitro with no collagen or Matrigel overlay.
- Extensive anastomosing networks of bile canaliculi with tight and gap junctions were formed by PHHs in the TCS.
- PHHs in the TCS remained functionally stable for over 22 days when Alb and urea levels were determined.
- There was significantly higher Alb, urea, and CYP activity in the PHHs in the TCS compared to PHHs in the SC monoculture.
- CYP- and Uridine 5'-diphospho-glucuronosyltransferase (UGT)-based enzyme activity and nuclear receptors were also stable during this time.
- The TCS represents a convenient, stable, and reproducible platform for pharmacological and toxicological applications in drug development and risk assessment.
References
LeCluyse, E.L., Witek, R.P., Andersen, M.E., Powers, M.J., 2012. Organotypic liver culture models: Meeting current challenges in toxicity testing. Crit Rev Toxicol. 42 (6), 501- 548.
Olson, H., Betton, G., Robinson, D., Thomas, K., Monro, A., Kolaja, G., et al., 2000. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 32 (1), 56-57.
Khetani, S.R., Bhatia, S.N., 2008. Microscale culture of human liver cells for drug development. Nat Biotechnol. 26 (1), 120-6.
Ware, B.R., Durham, M.J., Monckton, C.P., Khetani, S.R., 2017. A Cell Culture Platform to Maintain Long-Term Phenotype of Primary Human Hepatocytes and Endothelial Cells. Cell Mol Gastroenterol Hepatol. 5 (3), 187-207.
