Abstract
Thyroid disruptive chemicals (TDCs), including heavy metals and pesticides, may modify the functions of the thyroid, inhibit thyroid hormone (TH) regulatory enzymes, or alter TH levels in the blood or tissues leading to neurotoxicity. Data collected from animal TDCs studies are sometimes unreliable due to species differences in TDCs’ modes of action (MOA). However, immortalized human thyroid cell lines typically lose key thyroid specific functions and/or follicular epithelial architecture when cultured in 2D monolayers. No available primary or normal thyroid cell lines exhibit measurable levels of TH synthesis to screen TDCs. Currently, there is an unmet need for a reliable in vitro human thyrocyte model to investigate TDC-induced thyroid toxicity. In this study, our goal was to develop a bank of cryopreserved primary human thyrocytes qualified for thyroglobulin (TG) and thyroxine (T4) production that can serve as a model for chemical screening. Primary human thyroid cells were isolated from healthy donors of both genders with an age of ≤55 and a body mass index of ≤35. After an initial growth period (72-96 hr) in T-75 tissue culture-treated flasks, the cells were collected and cryopreserved. Each thyrocyte lot was characterized for post-thaw viability, expression of thyroid specific markers cytokeratin 7 (CK7) and TG, ability to form 3D microtissues on Matrigel-coated 96-well culture plates, and production of TG and T4 following treatment with thyroid-stimulating hormone (TSH). The results showed >80% post-thaw viability and >90% retention of thyroid specific protein markers across all lots (N=8). Thyrocytes consistently formed 3D microtissues on Matrigel and, on average, produced >4000ng/ml/well TG and >5ng/ml/well T4 7 and 14 days post-seeding, respectively. In conclusion, cryopreserved primary human thyrocytes produced robust and reproducible levels of key thyroid markers and could represent a valuable tool to prioritize TDCs and reveal key differences in MOAs between species.
Introduction
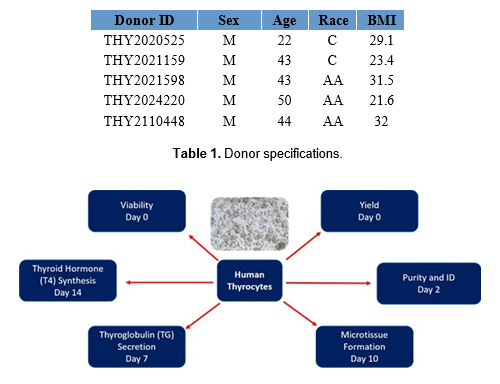
The purpose of this study was to validate cryopreserved primary human thyrocytes (PHT) which are isolated from normal adult donor tissues at LNH in a 96-well 3D microtissue format as an in vitro model to determine effects of potential TDCs on various T4-synthetic pathways. The focus on effects of TDCs has been increasing over the past 20 years and EPA urges the development of reliable and relevant non-animal, new approach methods. Due to extensive passage in culture and tumor specific origin, the current immortal thyroid cells typically do not retain the essential characteristics of native thyroid function and signaling pathways. Therefore, there is a need to develop an in vitro primary human thyrocyte model isolated from normal thyroids for TDC screening.
Materials and Methods
Isolation of Human Thyroid Follicular Epithelial Cells
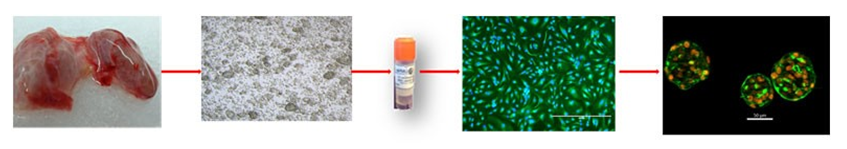
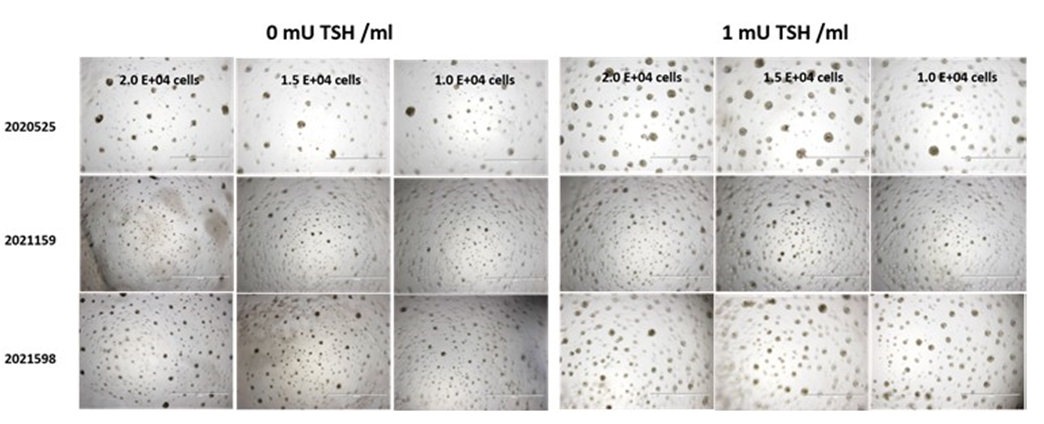
Thyrocytes were isolated from healthy thyroid tissues as previously described (1) and cryopreserved using CryoMed controlled-rate freezers. Monolayer cultures and 3D thyroid microtissues were prepared with cryopreserved primary PHT on TC-treated and Matrigel® (Corning) coated 96-well plates respectively. The 3D microtissue morphology of each batch was assessed over a 14-day period.
Treatments and Assays
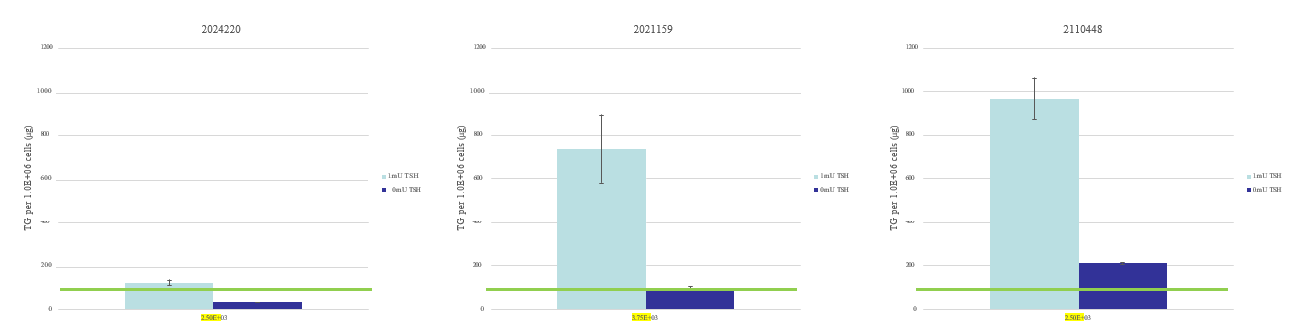
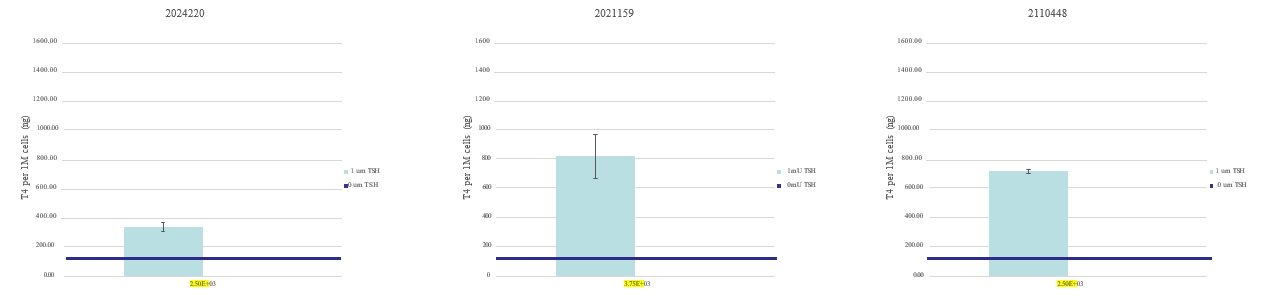
The thyroid microtissues were stimulated with 0mU/mL and 1mU/mL bovine thyroid stimulating hormone (TSH) (Sigma) from days 2-14. Expression of KRT7 and TG were assessed by immunocytochemistry with antibodies from Dako and Santa Cruz respectively. 4′,6-diamidino-2-phenylindole(DAPI) nuclear counterstain was used to demonstrate DNA content and nuclei. TG secretion and T4 synthesis were measured at select time points in collected media samples using ELISA kits from Invitrogen. The readouts from analytical assays were normalized to 1M cells. The minimum specifications for TG and T4 levels were determined by using reference values from a previous study (1)
Results
Conclusions
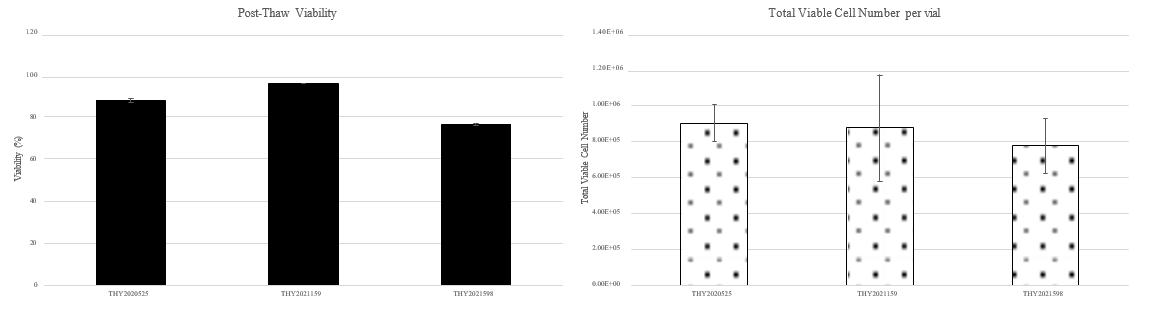
- ~80% of the recovered PHT are viable at post-thaw.
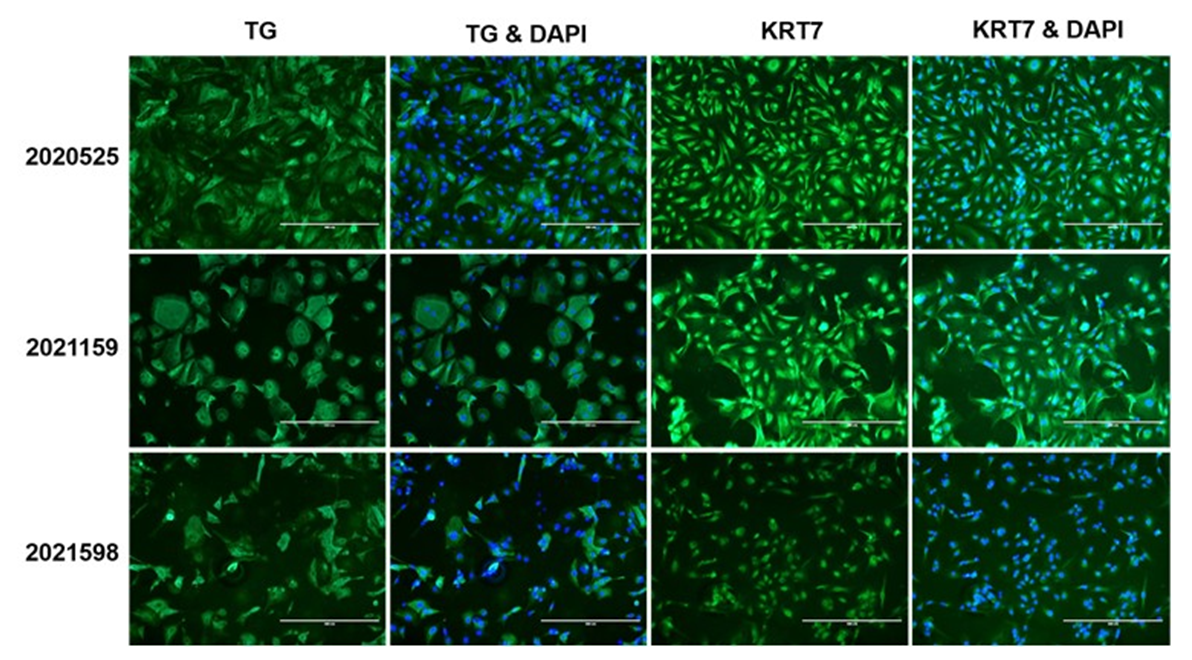
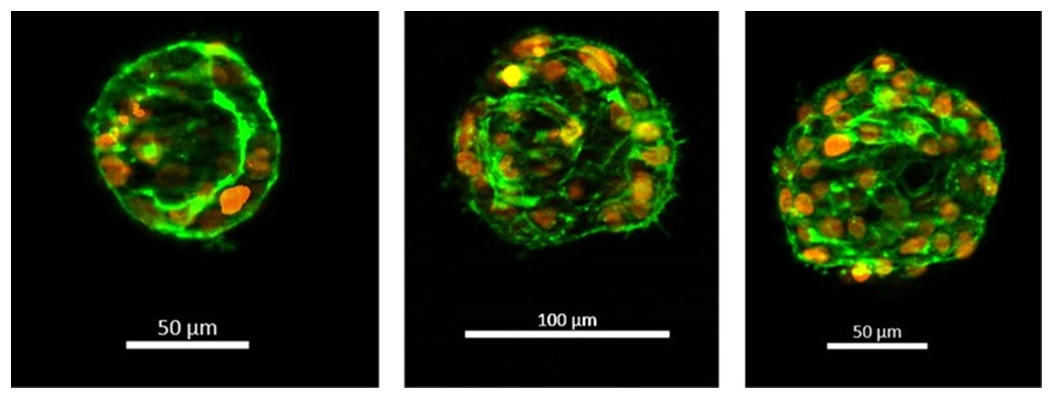
- KRT7 and TG expressions confirm that cryopreserved PHT retain thyroid follicular epithelial cell markers.
- Cryopreserved PHT form 3D thyroid microtissues with follicular-like morphology ( 50µm-150µm).
- TSH promotes 3D microtissue formation, TG secretion and T4 synthesis.
- Cryopreserved PHT maintain native thyroid function; TG secretion and T4 synthesis.
- Cryopreserved PHT represent a useful experimental tool for the screening of TDCs.
References/Acknowledgments
- Deisenroth C et al. (2019) Development of an in vitro human thyroid microtissue model for chemical screening. Toxicol Sci 174: 63-78.
- ECHA and EFSA, et al. (2018) Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009, EFSA Journal, 16 (2018), Article e05311.
