Kupffer cells (KC) constitute the largest population of liver macrophages, representing 80-90% of the total tissue macrophages in the body. As resident liver macrophages, KCs are a component of the walls of the hepatic sinusoids. These KCs allow the parenchymal cells in the liver (hepatocytes) to have selective access to fluid and protein in the blood through phagocytosis — the process of engulfing bacteria, microorganisms, and toxic agents carried in the blood. KCs play several roles during host defense mechanisms and liver inflammation.
First, they are activated and recruit other inflammatory cells to the site of injury by releasing a variety of chemokines and cytokines, such as tumor necrosis factor (TNF-α), interleukin (IL) -1β (IL-1β), IL-6, and IL-8. Second, KCs play an important role in the metabolic function of the liver by mediating the acute-phase response in hepatocytes and regulating the expression of cytochrome P450 enzymes (CYPs) and transporter proteins, which then influence the clearance and elimination of xenobiotics (foreign chemical substances). Third, the activation of KCs results in the production of reactive oxygen species (ROS), which then invoke cell death mechanisms during drug-induced liver injury (DILI). Lastly, KCs also play a role in liver fibrosis by activating hepatic stellate cells through cytokine secretion.
Isolated primary KCs have always been in high demand in liver research because of their many functions in the liver. The most traditional isolation method is to compile a supernatant of non-hepatocyte cells from freshly isolated human liver cells and obtain the KC-containing fraction at the interface of two different density gradients after centrifugation. Additional isolation can be done by allowing the cells to adhere to a tissue culture plate; the KCs will adhere, while the other nonparenchymal cells do not. 1 The KCs can then be identified by their two most prevalent biomarkers: CD11b and CD68.
As the formation of ROS is one of the most recognized cell death mechanisms in DILI, the mechanics of cytotoxicity in drug-induced injury, such as acetaminophen-induced injury, continue to be a highly relevant area of study in hepatotoxicity research. 2 The role of KCs in liver disease is complex. The current theory is that there are two main sets of KCs: a) resident KCs and b) bone marrow-derived KCs.3 Under healthy conditions, resident KCs are present in the liver as outlined above. However, during liver disease, bone marrow-derived macrophages rush to the liver and become resident KCs. Primary human KCs are an important tool for researchers to study liver disease and toxicity.
While primary isolated KCs alone are used in research, they are also widely applied to co-culture models such as 3D culture, and human organ on-a-chip (OOAC) systems. In these multicellular assays, they are used alongside hepatocytes, stellate cells, and endothelial cells. These systems are more physiologically relevant than the higher-throughput systems such as cell line- and rodent-based models.
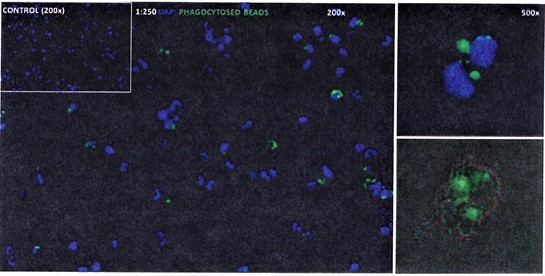
LifeNet Health LifeSciences provides high-quality primary human KCs that are isolated and then directly cryopreserved without culturing. The human KCs show phagocytosis function through phagocytosed-bead activation demonstrated in the images below. The function of cytokine secretion is tested through LPS induction in every lot of KCs provided by LifeNet Health LifeSciences.
References:
- Kegel, V., Deharde, D., Pfeiffer, E., Zeilinger, K., Seehofer, D. and Damm, G. Protocol for isolation of primary human hepatocytes and corresponding major populations of non-parenchymal liver cells. J Vis Exp. 2016 Mar 30;(109):e53069. DOI: 10.3791/53069.
- Jaeschke, H., McGill, M.R. and Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012 Feb; 44(1):88-106. DOI: 10.3109/03602532.2011.602688.
- Roohani, S and Tacke, F. Liver injury and the macrophage issue: Molecular and mechanistic facts and their clinical relevance. Int J Mol Sci. 2021 Jul 6;22(14): 7249. DOI: 10.3390/ijms22147249.
Authored by Huimin Yan, PhD. and Joel LeCluyse
