In Vitro Dermal Irritation Assay
Assessing the risk of dermal irritation, or the production of reversible damage of the skin following the exposure to a substance or mixture, is a primary consideration in determining the safety of topical drugs, chemicals or cosmetics as well as tin the cases of accidental exposures to chemicals, formulations, and other products.
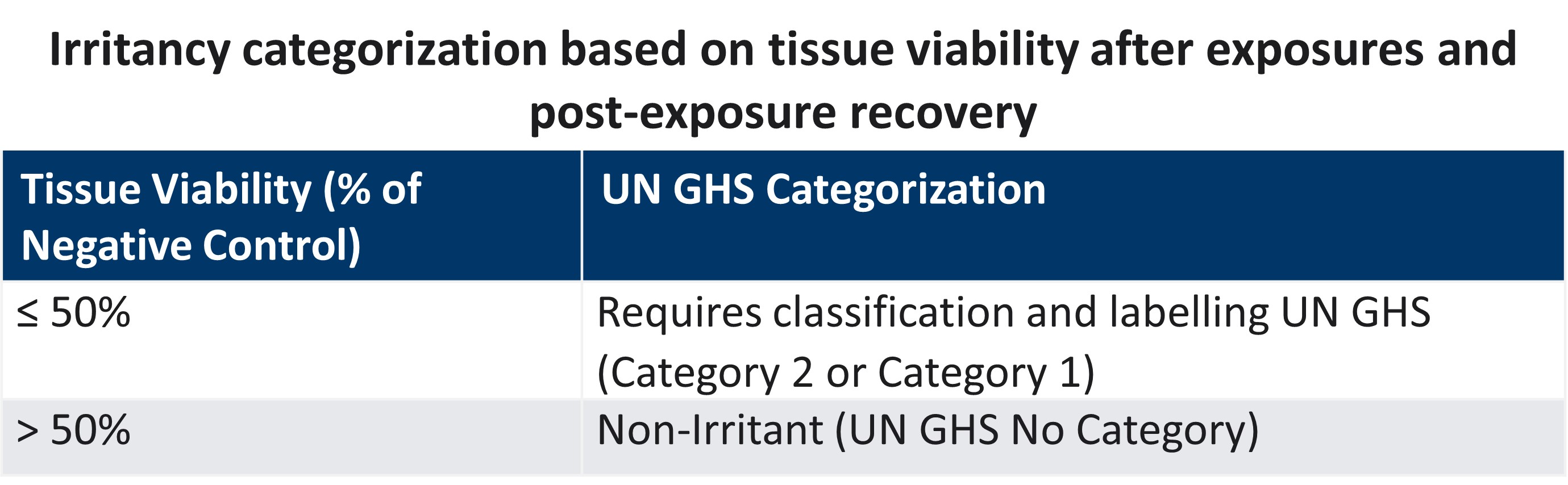
Dermal irritation testing combined with dermal corrosion testing can provide full UN GHS categorization of the chemical.
The Importance of Early Dermal Testing
- Risk Identification: Early assessment can determine a product's relative risk for causing dermal irritation.
- Early Identification: Initiation testing may identify chemicals (substances and mixtures) not requiring classification and labeling for skin irritation.
How LifeNet Health LifeSciences Can Help
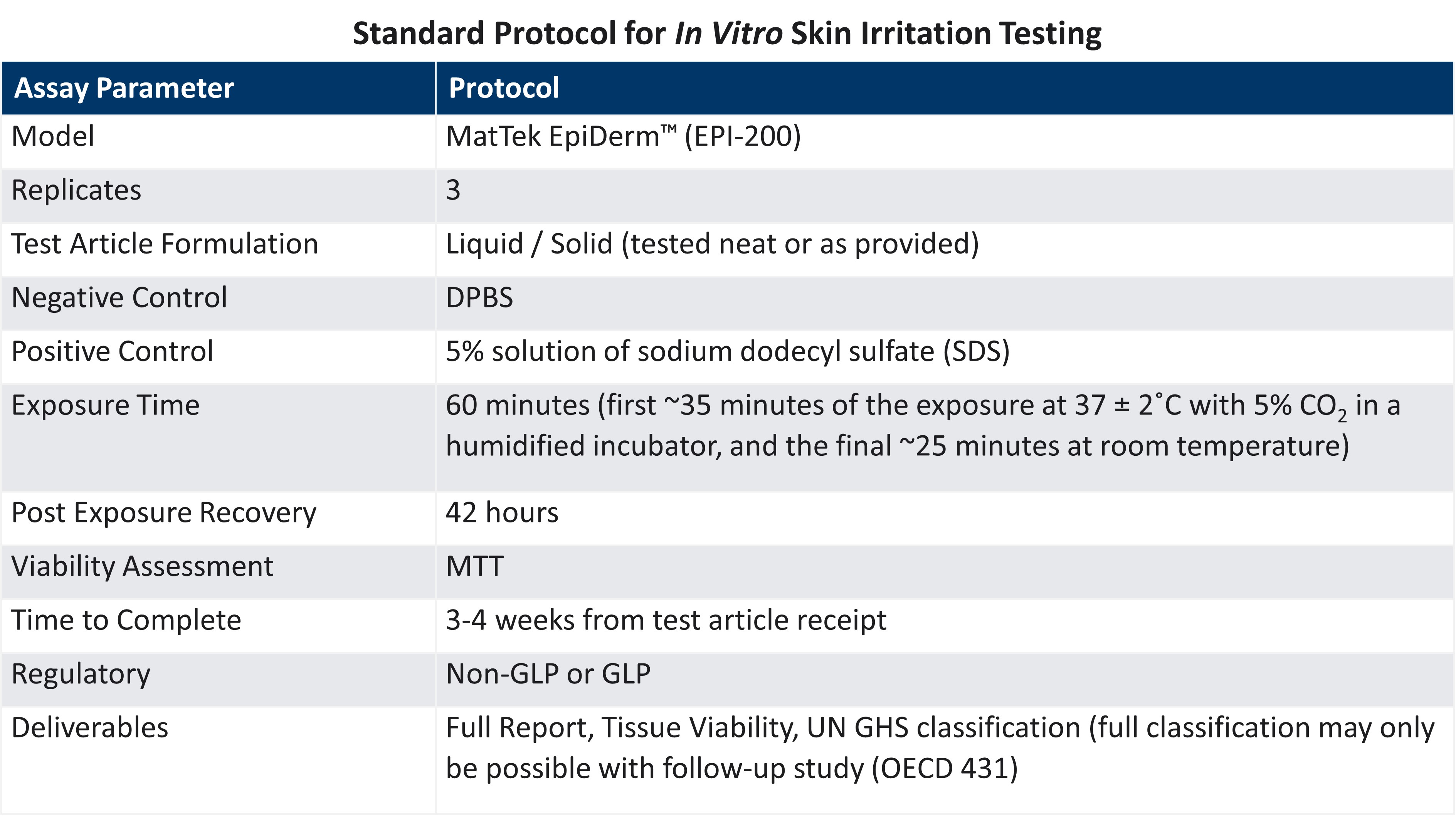
LifeNet Health offers chemical testing services with the validated EpiDerm™ SIT for the assessment of potential dermal irritation of the client's test articles, operating in full compliance with the OECD 439 guideline. Test compounds are applied topically to the EpiDerm SIT model using the basic procedures outlined below, and the health of the dermal tissue is assessed by measuring tissue viability immediately following exposure and after a post-treatment incubation period.
Benefits of Our Services:
- Accurate and reliable data for informed decision-making
- Fast turnaround times to keep your drug development on track
- OECD method-based studies to meet regulatory requirements
- Collaborative approach to ensure the study addresses your research questions
Connect with an expert
