CYP Inhibition Assay
As Cytochrome P450 (CYP) enzymes are the primary drug metabolizing enzymes in the body, they play a significant role in the metabolic clearance of drugs. CYP inhibition occurs when a new drug inhibits the metabolism of other drugs.
Why CYP Inhibition Matters
If CYP inhibition occurs it can cause:
- Increase drug concentrations in plasma and tissue
- Reduction of drug clearance
- Increased drug toxicity
FDA Guidance Based Methods – Direct and Indirect Inhibition
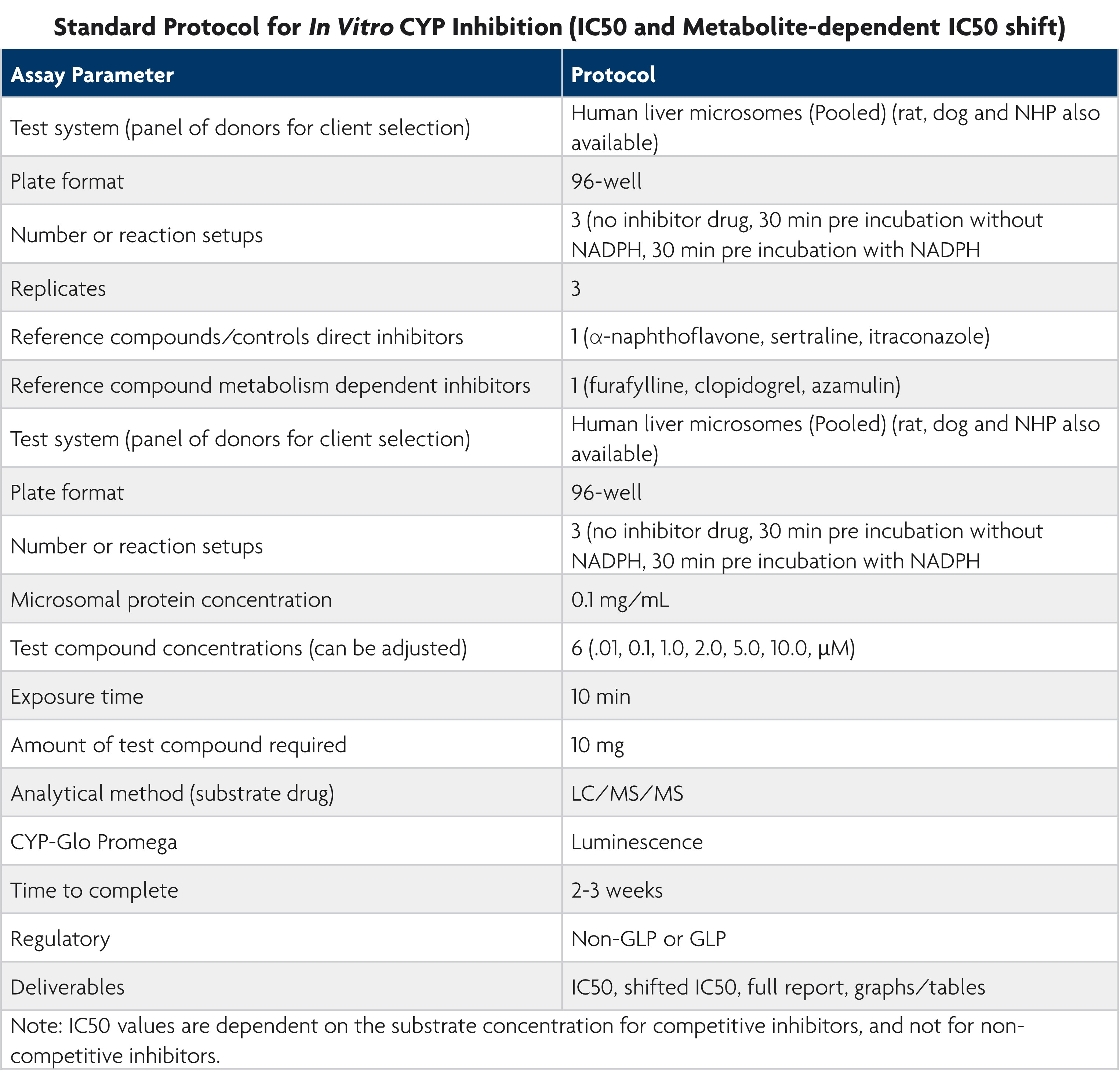
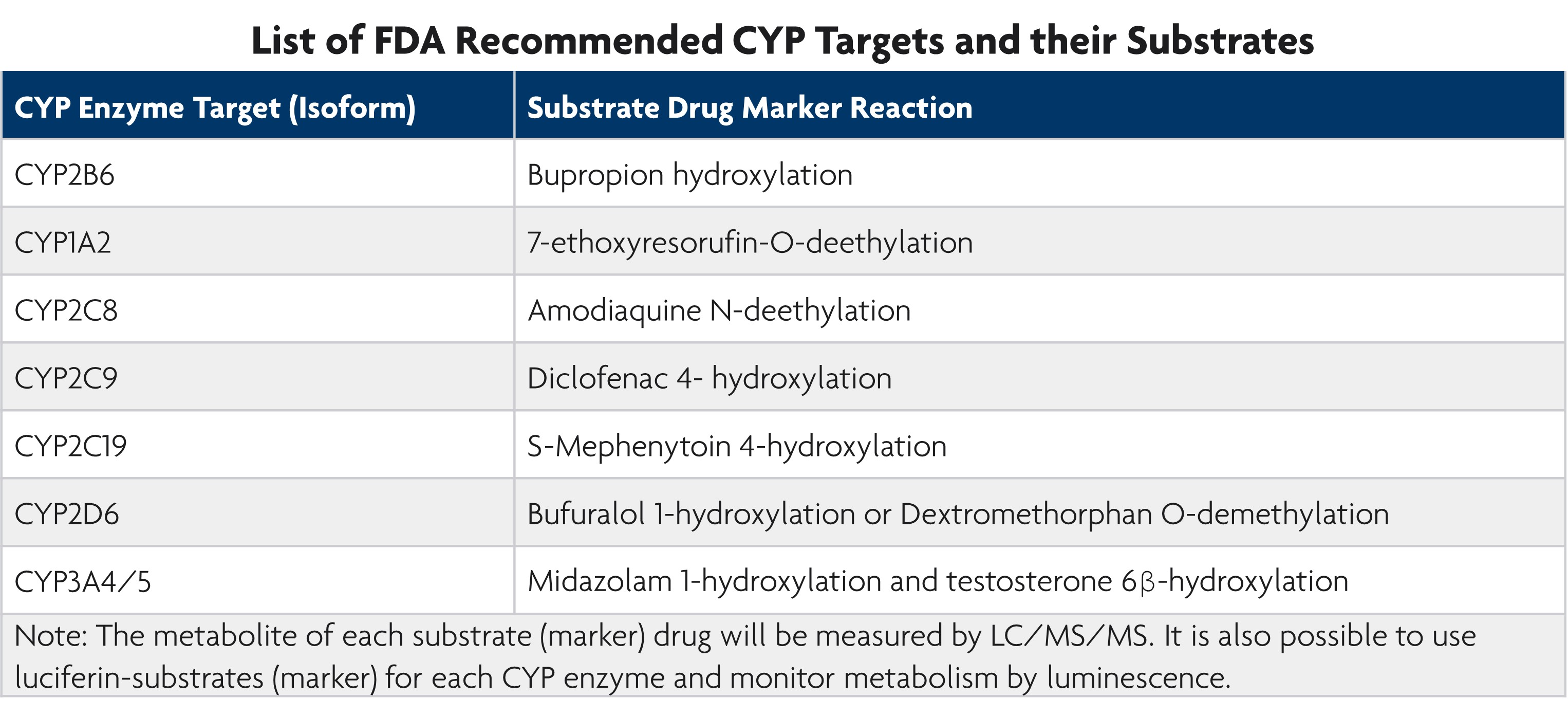
LifeNet Health LifeSciences Cell-Based Assays Services offers direct (IC50), indirect (IC50 Shift Assay), and time-dependent CYP inhibition assays based on the FDA Guidance document entitled, In Vitro Metabolism and Transporter Mediated Drug-Drug Interaction Studies.
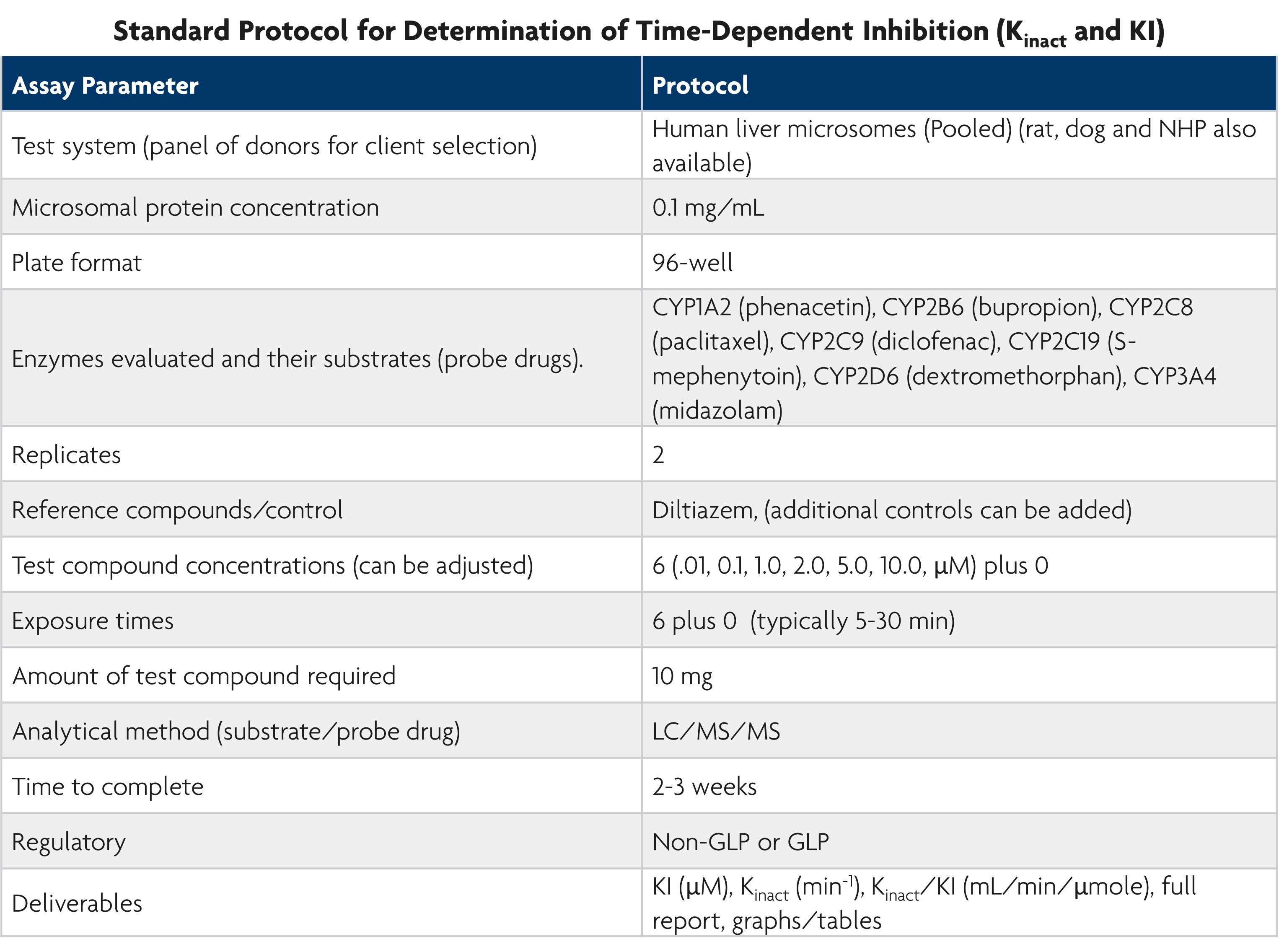
Direct inhibition is inhibition caused by the new chemical entity parent molecule while indirect inhibition refers to metabolite inhibition. Time-dependent inhibition determines the maximal rate of inactivation (Kinact) and the concentration that results in 50% Kinact during drug development.
Your Partner in CYP Inhibition Assays
Our services team offers a standardized protocol for assessing direct, indirect, and time-dependent CYP inhibition. This ensures high-quality data with the speed and accuracy needed for early drug discovery. We provide study designs suitable for IND submissions and collaborate with you to tailor the study to your specific research needs.
Benefits of Our Services:
- Accurate and reliable data for informed decision-making
- Fast turnaround times to keep your drug development on track
- FDA method-based studies to meet regulatory requirements
- Collaborative approach to ensure the study addresses your research questions