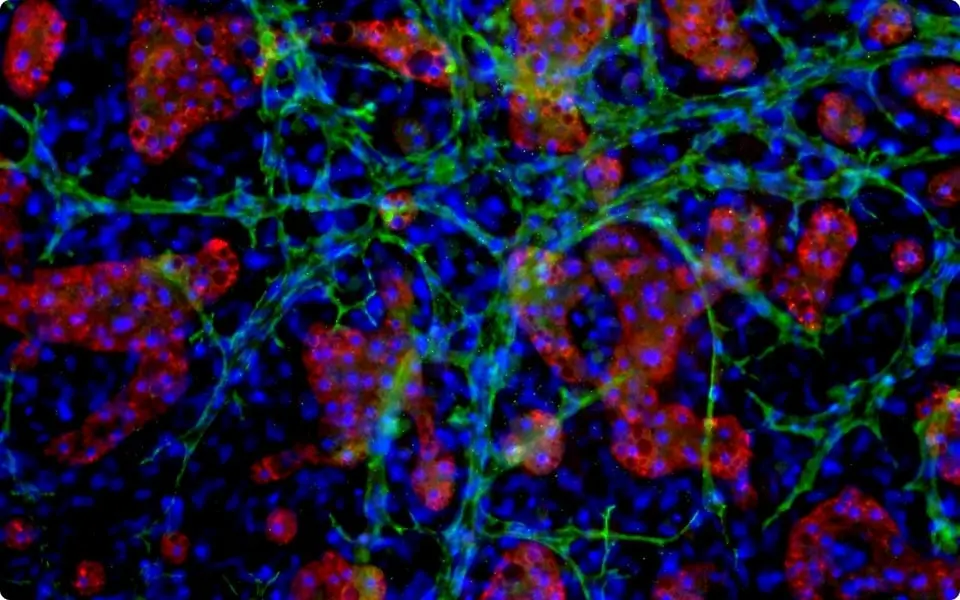
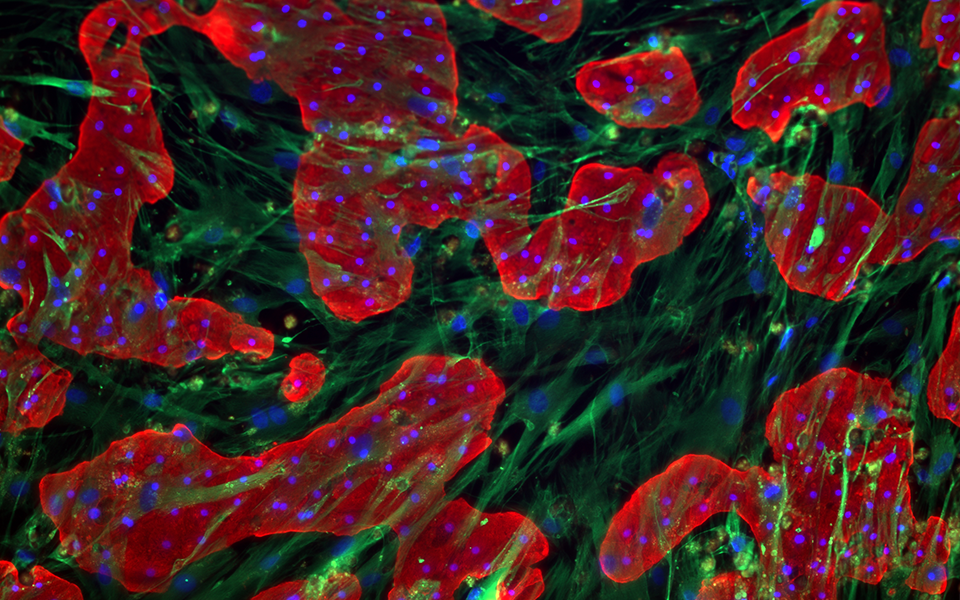
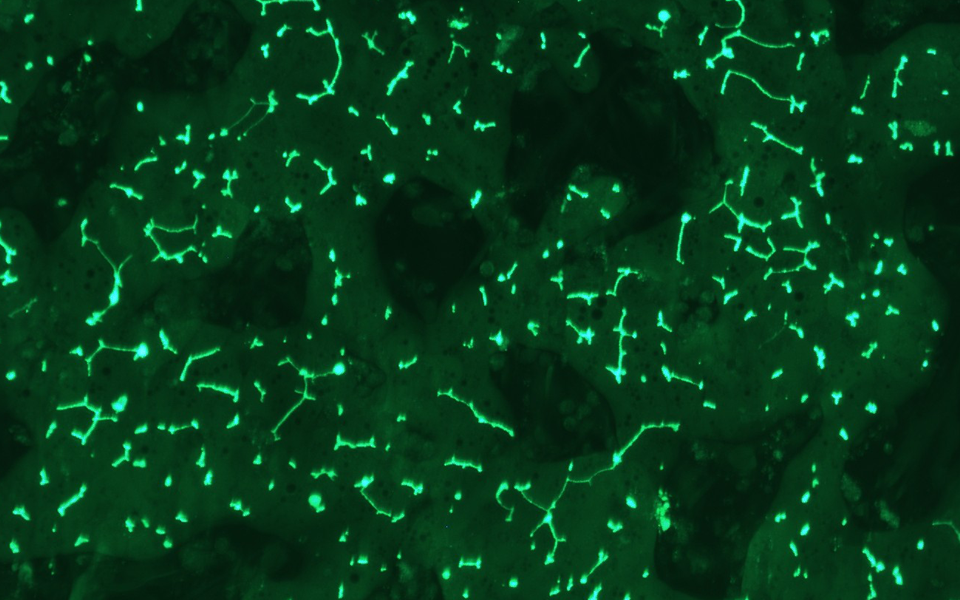
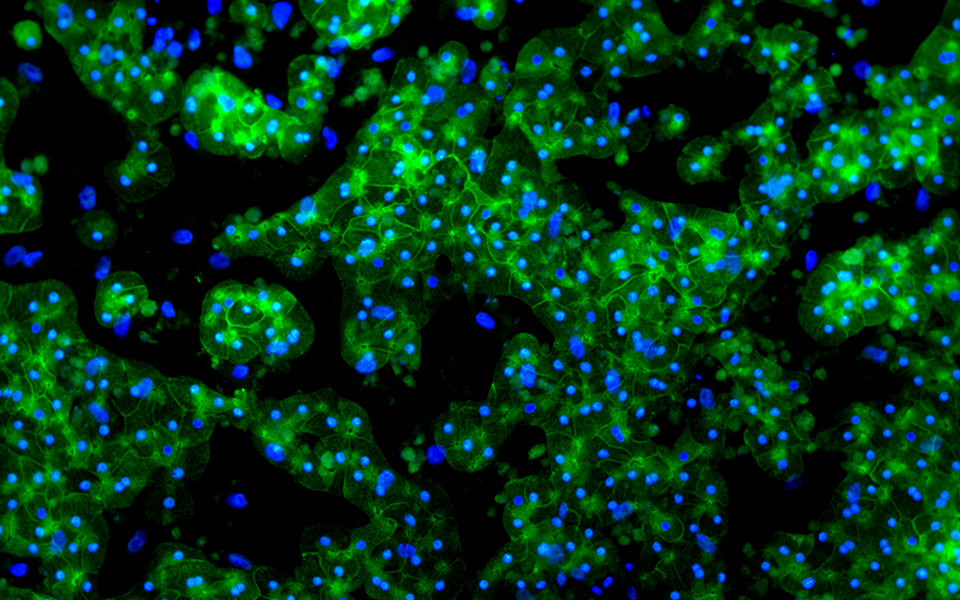
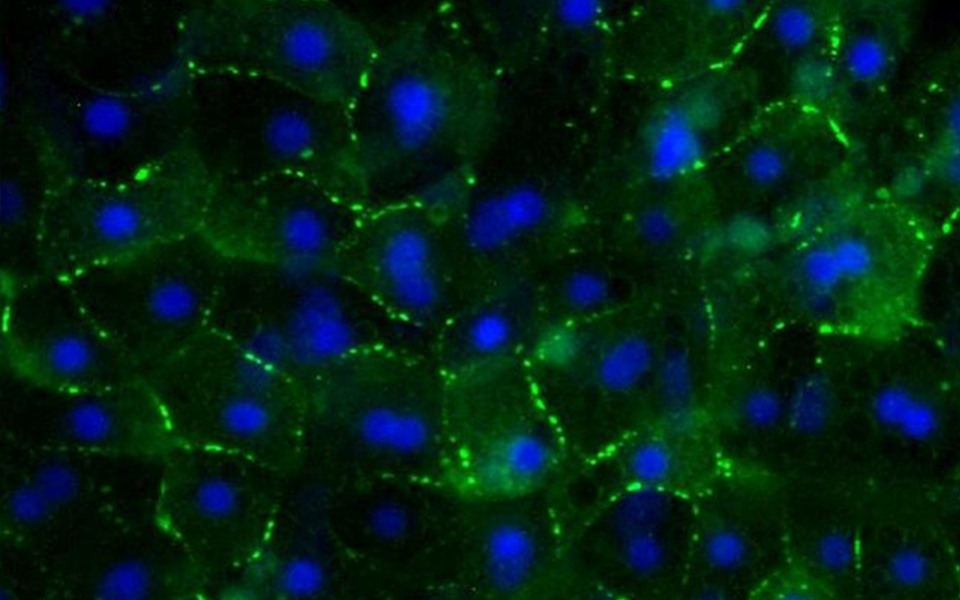
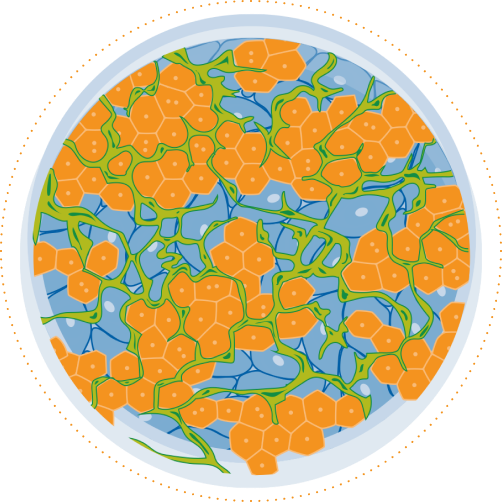
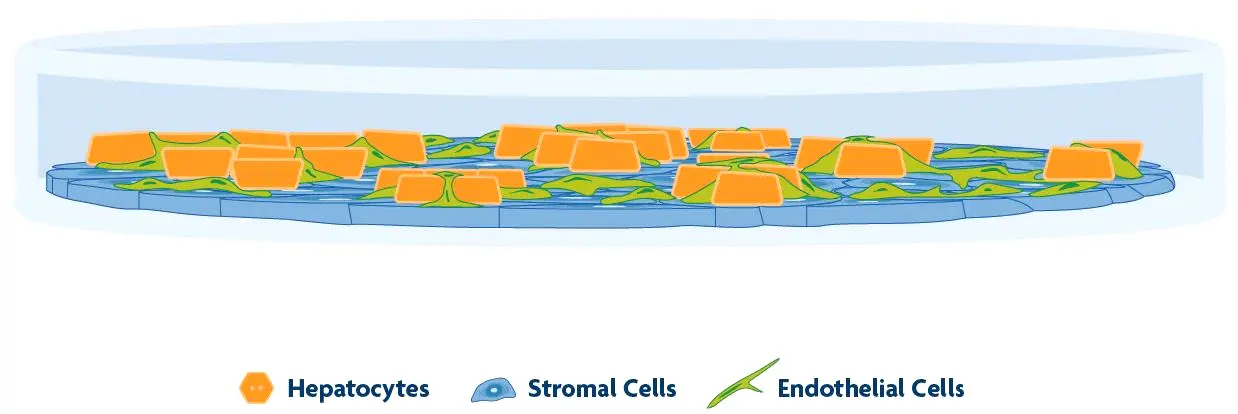
TruVivo® is the first and only 2D+ hepatic system to provide human-relevant, reliable results in an easy-to-use format. TruVivo kits include prequalified primary human cells combined at an optimized ratio to mimic the microarchitecture and functionality of the human liver.
For clients who want to leave the work to us, TruVivo is also available as a service - an option that offers true simplicity and ease. Our team of expert scientists can use TruVivo in cytotoxicity studies, ADME/DMPK assays, or to represent the liver in our multi-organ platform screening service.
Learn more about how TruVivo can be used in your research and see detailed performance data in our brochure.
The TruVivo kit includes prequalified cryopreserved primary human hepatocytes, primary human feeder cells, and optimized media components to support at least two weeks in culture.
Same- or next-day shipping is offered to most locations worldwide. Cells are shipped in the vapor phase of liquid nitrogen. Media components are shipped on dry ice or refrigerated cold packs.
TruVivo is available in 24-well and 96-well formats. Complete, ready-to-use kits and negative control kits (containing feeder cells and necessary media) are available.

TruVivo is an ideal platform for applications that require prolonged or repeated compound exposures, including the clearance of low-turnover compounds. Validation work by pharmaceutical partner, Boehringer Ingelheim, has demonstrated the potential for TruVivo to predict in vivo clearance. To view their poster, click here.
The feeder cells are not liver-derived but are a mixture of primary human stromal and endothelial cells.
For each format, 24- and 96-well, the ratio of feeder cells to hepatocytes has been optimized to achieve human-relevant hepatocyte morphology and functionality. Optimal seeding densities for hepatocytes and feeder cells are provided.
The feeder cells have been growth-arrested to minimize cell expansion.
The total number of attached hepatocytes per well is lot-dependent. Based on initial seeding density, expect greater than or equal to 50% attachment. The calculated hepatocyte cell number per well for each lot is provided for data normalization. For more information about how we calculate this value, click here.
Kits include enough media to accommodate daily medium changes for two weeks − however, the frequency of medium changes can be adapted to meet specific assay needs. For most culture work, it is recommended to change at least every two to three days. For applications that require prolonged compound exposures, plates can go up to seven days without changing the medium.
Typically, by day five, cell morphology and functionality have stabilized, and cells are ready for compound treatment. This can be extended to seven days if needed for workflow convenience.



CELL SOLUTIONS
LifeNet Health’s portfolio of pioneering all-human cell solutions are leading the way in human in vitro biology. Connect with our team of experts to discover how our cutting-edge cell solutions can work for your research applications.